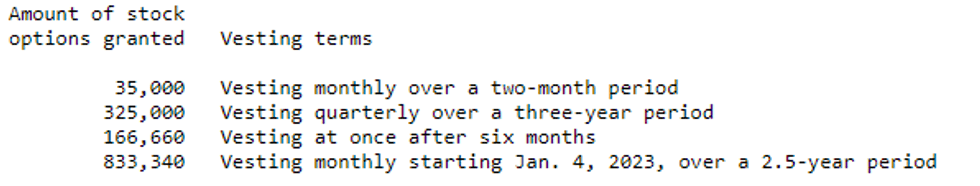July 04, 2022
BioHarvest Sciences Inc. ("BioHarvest" or the "Company") (CSE: BHSC) announces that it has granted a total of 1,360,000 stock options to consultants and employees. The stock options granted are exercisable to purchase a common share of the Company at a price of $0.24 per share for a term of 10 years and will vest as follow-:
Dr. Zaki Rakib, Director of the Company, has agreed to cancel a total of 4,343,800 stock options previously issued to him on March 23, 2021. The Company now has more available options to attract and retain future employees, consultants, directors, and officers of the Company by affording them the opportunity to acquire an interest in BHSC through options granted under the Incentive Option Plan.
About BioHarvest Sciences Inc.
BioHarvest Sciences Inc. (CSE: BHSC) is a fast-growing Biotech firm listed on the Canadian Securities Exchange. BioHarvest has developed a patented bio-cell growth platform technology capable of growing the active and beneficial ingredients in fruit and plants, at industrial scale, without the need to grow the plant itself. This technology is economical, ensures consistency, and avoids the negative environmental impacts associated with traditional agriculture. BioHarvest is currently focused on nutraceuticals and the medicinal cannabis markets. Visit: www.bioharvest.com.
BioHarvest Sciences Inc
Ilan Sobel, Chief Executive Officer
For further information, please contact:Dave Ryan, VP Investor Relations & DirectorPhone: 1 (604) 622-1186Email: dave@bioharvest.com
Forward-Looking Statements Information set forth in this news release includes forward-looking statements that are based on management's current estimates, beliefs, intentions, and expectations, and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. There is no assurance that we will achieve our objective of being a leading supplier of Cannabis. Delays and cost overruns may result in delays achieving our objectives. Projected sales of Cannabis will require the company to obtain production and / or export licensing which cannot be assured.
All forward-looking statements are inherently uncertain and actual results may be affected by a number of material factors beyond our control. Readers should not place undue reliance on forward-looking statements. BHSC does not intend to update forward-looking statement disclosures other than through our regular management discussion and analysis disclosures.
Neither the Canadian Securities Exchange nor its Regulation Services Provider accept responsibility for the adequacy or accuracy of this release.
Click here to connect with BioHarvest Sciences Inc. (CSE: BHSC) to receive an Investor Presentation
BHSC:CNX
The Conversation (0)
02 January 2022
BioHarvest Sciences
Developing Disruptive Biofarming Technology for the Cannabis Industry
Developing Disruptive Biofarming Technology for the Cannabis Industry Keep Reading...
Latest News
Interactive Chart
Latest Press Releases
Equity Metals Exhibiting at the 2026 PDAC
06 February
Related News
TOP STOCKS
American Battery4.030.24
Aion Therapeutic0.10-0.01
Cybin Corp2.140.00