Medtronic and Harris Poll survey finds 4 in 5 report chronic back or leg pain is worse or unimproved since the COVID-19 pandemic began
A new survey commissioned by Medtronic plc (NYSE:MDT), a global leader in healthcare technology, and conducted by public opinion research firm The Harris Poll, finds nearly half (44%) of current chronic back and leg pain sufferers have experienced care delays during the COVID-19 pandemic, despite 87% reporting that their pain has not improved or even worsened since the pandemic began in March 2020 .
The national survey, "Painful Pandemic: How a Healthcare System Under Strain Impacts Chronic Pain Patients," of 810 U.S. adults who currently experience chronic back or leg pain finds far-reaching impacts of this debilitating condition on patient lifestyle, everyday activities, and mental state, further exacerbated by the pandemic's impact on stressed health systems and the ability of patients to seek timely care.
Of those who report worsening chronic pain during the pandemic, more than half (52%) cite challenges in receiving appropriate medical care as a contributing factor. Additionally, 44% say since the pandemic began, they have experienced care delays, including postponed, rescheduled, or canceled appointments or procedures for their pain. Of those who proactively postponed their medical care, more than half (55%) cited COVID-19 fears as a contributing factor.
Pain significantly impacts daily life
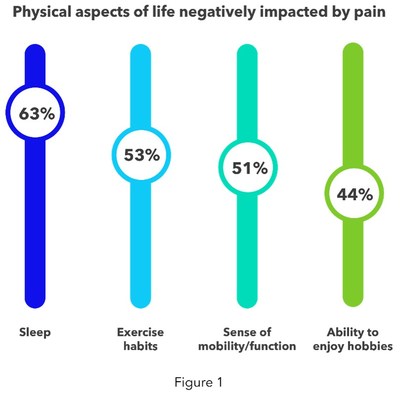
Most people currently living with chronic back or leg pain report numerous detrimental physical and mental impacts. When listing physical challenges associated to living with chronic pain, sleep, exercise habits, sense of mobility/function, and ability to enjoy hobbies are aspects of life most negatively impacted (see figure 1). Chronic back or leg pain sufferers under age 55 are more likely than those 55+ to report that their ability to work has been impacted (36% vs. 22%, respectively), while those over age 55 are more likely than those age 35-54 to say their sense of mobility/function has been impacted (58% vs. 46%, respectively).
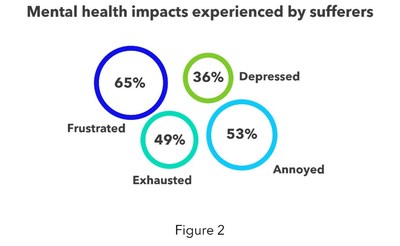
In addition, most (66%) report that their mental health has been negatively impacted and list feelings of frustration, annoyance, exhaustion, and depression (see figure 2). Surveyed patients also say that it is harder to enjoy spending time with their family (67%).
As a result, the overwhelming majority (90%) of sufferers say they wish there were more treatment options available to manage their pain. While most are aware of traditional treatment options like physical therapy and oral medications, far fewer are familiar with options such as targeted drug delivery (38%) or spinal cord stimulation (34%). On average, those who have seen or talked to a healthcare provider, about their chronic back or leg pain, first did so seven years ago, while 38% of current chronic back or leg pain sufferers say they have never been referred to a pain specialist physician for their chronic pain.
"This data bears out what we've heard from our clinician customers and patients for two years – the pandemic has been especially hard on those with chronic pain," said Charlie Covert , vice president and general manager, Pain Therapies within the Neuromodulation business, which is part of the Neuroscience portfolio at Medtronic. "There is a tangible human cost to deferred procedures and delayed care. As COVID-19 hopefully begins its transition to a more endemic disease, we expect many of these patients to urgently seek relief through new or more effective treatment modalities. Our survey demonstrated that an overwhelming majority want more treatment options, yet awareness of spinal cord stimulation and targeted drug delivery options remains relatively low. That represents an opportunity to educate patients about the full range of options available to help alleviate their pain."
For more information about the survey, visit https://www.medtronic.com/us-en/c/pain-therapies/pain-study-results.html .
About this survey
This survey was conducted online within the United States by The Harris Poll on behalf of Medtronic among 810 U.S. adults ages 18+ who currently experience chronic back or leg pain. This online survey is not based on a probability sample and therefore no estimate of theoretical sampling error can be calculated.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland , is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn .
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
| Contacts: | |
| | |
| Jeff Trauring | Ryan Weispfenning |
| Public Relations | Investor Relations |
| +1-763-505-0159 | +1-763-505-4626 |
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/medtronic-survey-finds-pandemic-creating-far-reaching-and-negative-impacts-on-those-with-chronic-back-or-leg-pain-301508543.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/medtronic-survey-finds-pandemic-creating-far-reaching-and-negative-impacts-on-those-with-chronic-back-or-leg-pain-301508543.html
SOURCE Medtronic plc

![]() View original content to download multimedia: https://www.newswire.ca/en/releases/archive/March2022/23/c7490.html
View original content to download multimedia: https://www.newswire.ca/en/releases/archive/March2022/23/c7490.html