Geomega Resources’ Test Series Confirm Simultaneous Separation of Heavy REE and Light REE
Geomega Resources Inc. (TSXV:GMA) released results of tests confirming physical separation of neighbour rare earth elements based on free flow electrophoresis technology. This series of tests concluded simultaneous separation of selected heavy REE and light REE, and separation of neighbour REE such as Dysprosium/Terbium and Neodymium/Praseodymium. The tests also show that the free flow electrophoresis technology attains multicomponent separation of REE in a single pass.
As quoted in the press release:
Five (5) sets of tests using different separation protocols have been selected for the neighbour series. They consist of three (3) multi-element tests (A, B and C) associated with two complementary binary tests (B+ and C+).
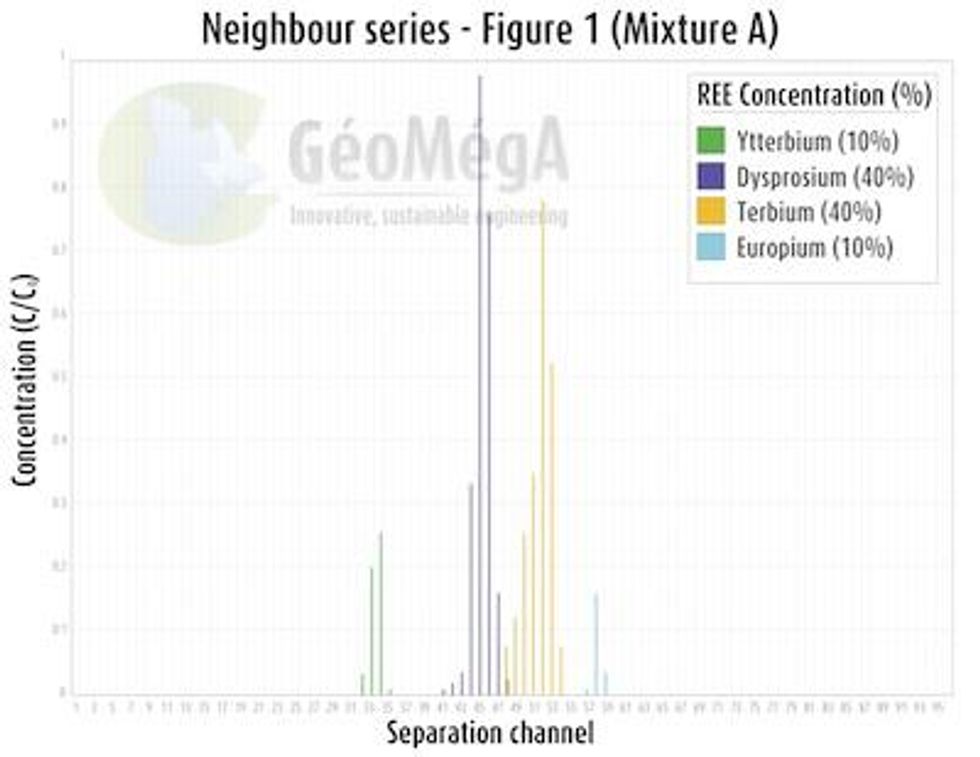
Synthetic mixture A contains Ytterbium (Yb), Dysprosium (Dy), Terbium (Tb) and Europium (Eu) representing the heavy to medium REE;
Synthetic mixture B contains Europium (Eu), Neodymium (Nd), Praseodymium (Pr) and Lanthanum (La) representing the medium to light REE;
Synthetic mixture B+ is the corresponding binary mixture to B, containing Pr and Nd. This result demonstrates the elemental isolation of neighbours (i.e. Nd and Pr) when they occur together in some channels during the first separation pass (see figure 2, channels 59 to 62);
Synthetic mixture C contains Europium (Eu), Praseodymium (Pr), Cerium (Ce) and Lanthanum (La) representing the light REE;
Synthetic mixture C+ is the corresponding binary mixture to C, containing Ce and Pr. This result demonstrates the elemental isolation of neighbours (i.e. Pr and Ce) when they occur together in some channels during the first separation pass (see figure 4, channels 64 to 69).
All tests have been performed in a single pass using a laboratory scale prototype equipment;
Total REE concentration is approximately 1 g/L (65 millimolar).The graphic results (CLICK HERE) highlight the following features of the technology:
Multicomponent separation of REE attained in a single pass;
100% purity separation of HREE is achievable in a single pass in the current process condition;
100% purity separation of medium to LREE is achievable in two passes in the current process condition;
100% purity separation of LREE is achievable in a continuous loop in the current process condition.
GéoMégA President and CEO, Simon Britt, said:
Physics, when understood correctly, is predictable. The perfect binary separation of Nd and Pr with the currently limiting prototype equipment highlights a development team in control. We will ballpark a development timetable after the next testing program is completed.
Click here to read the Geomega Resources Inc. (TSXV:GMA) press release
Click here to see the Geomega Resources Inc. (TSXV:GMA) profile.