Zafgen saw its share price almost double on Wednesday after the release of positive data on its experimental obesity drug.
It was a rough day in the markets, but a great one for biopharmacetical company Zafgen (NASDAQ:ZFGN).
The obesity treatment-focused company saw its share price nearly double on Wednesday after the release of positive data on its experimental obesity drug. The data could convince the US Food and Drug Administration (FDA) to lift a hold that was placed on Zafgen’s clinical trials in December following the death of two patients.
The company’s lead drug, Beloranib, can reportedly significantly reduce body weight and an excessive urge to eat in patients suffering from Prader-Willi syndrome, the most common genetic cause of life-threatening obesity. Beloranib is the first investigational drug that’s shown effectiveness in reducing body weight.
According to Reuters, Wednesday’s data shows that patients given a 2.4-milligram dose of Beloranib shed 9.45 percent of their body weight. At a 1.8-milligram dose, patients lost 8.2 percent of their body weight. The data comes from 74 patients who completed the late-stage study and 27 patients who completed at least 75 percent of the trial prior to the hold placed by the FDA.
“This clear efficacy outcome is a crucial first step in moving discussions forward with the Food and Drug Administration regarding continued development of beloranib,” Chief Executive Thomas Hughes said. “While we take the previously reported adverse events very seriously, we now have the robust data to provide greater perspective on the benefit/risk relationship of beloranib in this high-risk patient population.”
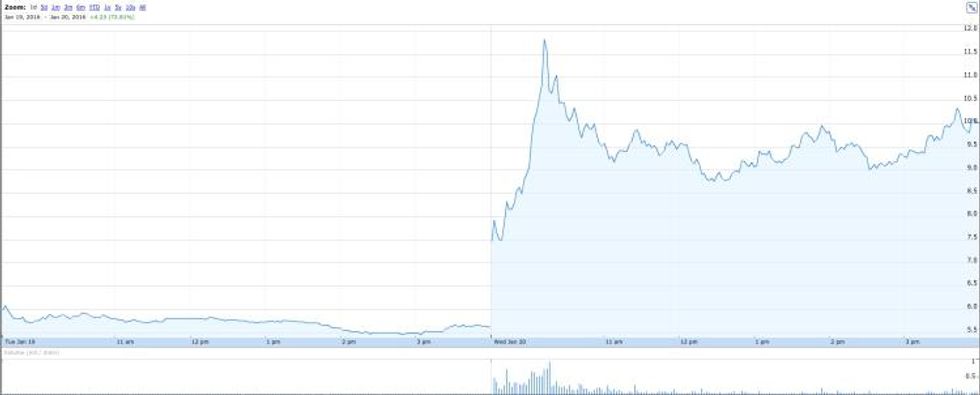
As mentioned, the news boosted the company’s share price, which suffered a massive hit in 2015 — it fell 84 percent between the week before the death of the first patient and this week. On Wednesday, the company saw its share price jump 78.65 percent, ending the day at $10.04.
It’s now only a matter of time before Zafgen hears back from the FDA.
Securities Disclosure: I, Vivien Diniz, hold no direct investment interest in any company mentioned in this article.

