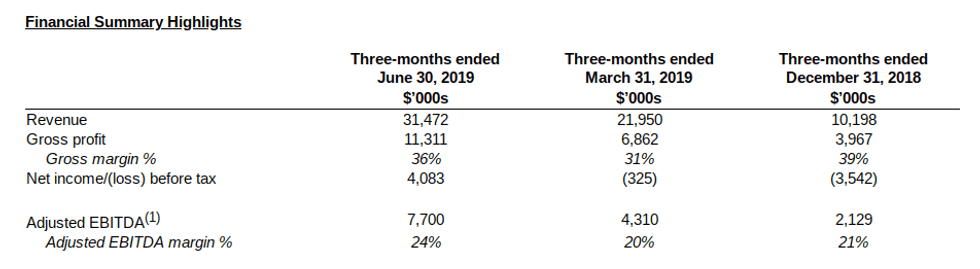MediPharm Labs Reports Strong, Profitable Second Quarter Results
Q2 2019 Revenue of $31.5 Million, Adjusted EBITDA of $7.7 Million, Net Income before tax of $4.1 Million
MediPharm Labs Corp. (TSX:LABS) (OTCQX:MEDIF) (FSE:MLZ) (“MediPharm Labs” or the “Company”) a global leader in specialized, research-driven cannabis extraction, distillation, purification and cannabinoid isolation, today announced its financial results for the three and six months ended June 30, 2019, including strong growth in revenue, EBITDA, net income and earnings per share.
KEY Q2 2019 HIGHLIGHTS(2)
- Revenue was $31.5 million, a 43% increase over Q1 2019, reflecting the Company’s leadership of the Canadian cannabis extraction-only industry and ramp up of new committed contracts
- Gross Profit was $11.3 million, a 65% increase over Q1 2019, while Gross Margin was 36% compared to 31% in Q1 2019, reflecting increased production and production efficiency that continues to improve as the Company realizes economies of scale
- Adjusted EBITDA(1) was $7.7 million, 79% higher than Q1 2019, while Adjusted EBITDA(1) margin was 24% compared to 20% in Q1 2019
- Net income before tax was $4.1 million compared to a net loss of $0.3 million in Q1 2019
- Gross proceeds of $75 million were received from an oversubscribed bought-deal offering of 13.5 million common shares at a price of $5.55 per share and $9.5 million proceeds from warrant exercises
- Investing capital raised to build additional scale in Canada and Australia that will support an increase in the Company’s addressable markets and further diversify its product lines in anticipation of expanded Canadian legalization in the fall of 2019
(1) See Non-IFRS Measures section of this news release.
(2) There is no prior-year comparable as the Company commenced production in the fourth quarter of 2018.
“MediPharm Labs made tremendous progress in the second quarter with growth in all key value drivers,” said Patrick McCutcheon, Chief Executive Officer, MediPharm Labs. “Based on the strength of our business model and the effectiveness of our corporate strategies, we’ve become the first public Canadian extraction only company to deliver bottom-line earnings and do so while investing heavily in our sophisticated extraction and production platforms ahead of benefits realized. The significant momentum we’ve gained through important new customer and procurement relationships, and the successful ramp up of our Canadian and Australian facilities will drive growth and value for the remainder of this year and well beyond.”
YEAR-TO-DATE 2019 HIGHLIGHTS
- Revenue for the six months ended June 30, 2019 was $53.4 million, gross profit was $18.2 million (gross margin 34%), Adjusted EBITDA(1) was $12.0 million (Adjusted EBITDA margin of 22%) and net income was $1.4 million ($0.01 per share basic and diluted)
- Cash balance of $72.7 million at June 30, 2019 compared to $7.9 million at December 30, 2019
- Secured an agreement with Cronos Group, May 13, 2019 for approximately $30 million of cannabis concentrate over 18-months, and, subject to certain renewal and purchase options, potentially up to $60 million over 24 months
- Canadian production increased to an average of 75 million MG of active cannabinoid component concentrate on a weekly basis at the end of June 2019
- Procured 9,000 KG of dried cannabis in June 2019 for process and sale in Q3 2019
- Secured dried cannabis from more than 23 Health Canada-compliant Licensed Producers since Q4 2018 that met MediPharm Labs’ enhanced quality control agreements
- Annual dried cannabis processing capacity in Canada increased to 300,000 KG and with a new customized, large-scale extraction line set to open later this year at the Company’s Barrie, Ontario headquarters, annual capacity is expected to increase to over 500,000 KG
- Commenced construction for 25,000 square feet of additional licensed space, subject to Health Canada approval, to accommodate significant automation to support filling, packaging and new product manufacturing, cannabinoid isolation activities and specialized R&D at the Company’s Barrie, Ontario headquarters
- Secured a white label agreement for a minimum of 2 million vape pens, subject to purchase orders, with AV Cannabis, the owner of the top-rated Ace Valley cannabis brand in Canada to supply high-quality cannabis extracts, filling services and national distribution for a new line of custom-formulated Ace Valley vape pens
- Marked the successful completion of the Company’s first international export shipment of medical cannabis concentrate to AusCann in Australia, authorized by import/export permits granted by the Australian Office of Drug Control and Health Canada, respectively
- Awarded a manufacturing licence and nearing completion of a 10,000 sq. ft. purpose-built facility southeast of Melbourne, Australia that will house supercritical CO2 extraction to process up to 75,000 kg of dried cannabis annually as well as state-of-the-art secondary processing equipment for the manufacture of purified and high-concentrate cannabis distillate
- Invested in the ramp up of industrial scale soft gel capsule capabilities and the foundation to become one of the largest vaporizer cartridge fill manufacturers in Canada
- Graduated to the Toronto Stock Exchange on July 29, 2019. A market open ceremony with MediPharm Labs is scheduled for Wednesday, August 14, 2019
NEAR-TERM CATALYSTS
- Fulfillment of large new customer agreements in process
- Expanded distribution through new supplier status with additional provincial government cannabis boards
- Ramp up of production and increases in capacity at Canadian and Australian extraction facilities
- Expected Canadian legalization of vapeables, topicals and edibles in fall 2019 which will add significantly to the addressable market for cannabis derivatives
- New higher margin product development: soft gel caps and vapourizer cartridges
- Traction with international growth strategy: expected licensing of MediPharm Labs Australia state-of-the-art cannabis extraction facility; expected European Union GMP certification of Barrie facility; and evaluation of partnership opportunities to address jurisdictions in Europe, Latin America, the Caribbean and South Africa
Corporate Update
- On April 30, 2019, the Company appointed medical expert and pharmaceutical researcher, Dr. Paul Tam as a new independent director on its Board of Directors. With more than three decades of clinical research and medical practice experience, Dr. Paul Tam is a globally recognized expert in the field of nephrology as well as bringing experience from his tenure with leading pharmaceutical companies
- The Company appointed former Johnson & Johnson Group Product Director, Braden Fenske as Chief Strategy Officer April 29, 2019. Mr. Fenske is responsible for advancing the Company’s strategic corporate initiatives in collaboration with the Company’s executives and operational teams across the company
- In addition, the Company announces the planned retirement of its Chief Operating Officer, David Mayers. As a result of growth in management capacity, the responsibilities formerly held by Mr. Mayers will be dispersed to Keith Strachan, President, Kirk Binns, Executive Vice President, and Braden Fenske, Chief Strategy Officer, along with other aspects of production leadership taken on by members of our growing team of more than 180 researchers, scientists, professionals and technicians
- As part of the Company’s continued policy of incentivizing its employee base with stock options, the Company granted 1,851,700 options to new and existing hires at an exercise price set as at the close of business on August 13, 2019. 300,000 of such options were granted to Dr. Paul Tam. Each grant has a five-year term expiring August 13, 2024, and vests in five equal instalments, the first of which vests immediately with the four other instalments vesting on the dates which are six, twelve, eighteen and twenty-four months from the grant date. The stock options were granted to directors, officers, employees and consultants of the Company and are subject to any necessary regulatory approvals
Q2 CONFERENCE CALL AND WEBCAST
The Company will host a conference call and audio webcast on Tuesday, August 13, 2019 at 9:30 a.m. eastern time to discuss its results and outlook. Participants are asked to dial in approximately 10 minutes before the start of the call using one of the following numbers: Toll-free: 877-791-0216 International: 647-689-5661.
An audio webcast will be available in the Events section of the MediPharm Labs’ Investor Relations website https://ir.medipharmlabs.com/news-events or by visiting the following link: https://event.on24.com/wcc/r/2061708/FA5DDF7A03088FC1E7183DBBA763DEB7
For those who are unable to participate on the live conference call and webcast, a replay will be available approximately one hour after completion of the call at: Toll-free: 800-585-8367 International: 416-621-4642. Please reference Conference ID: 2783678
NON-IFRS MEASURES
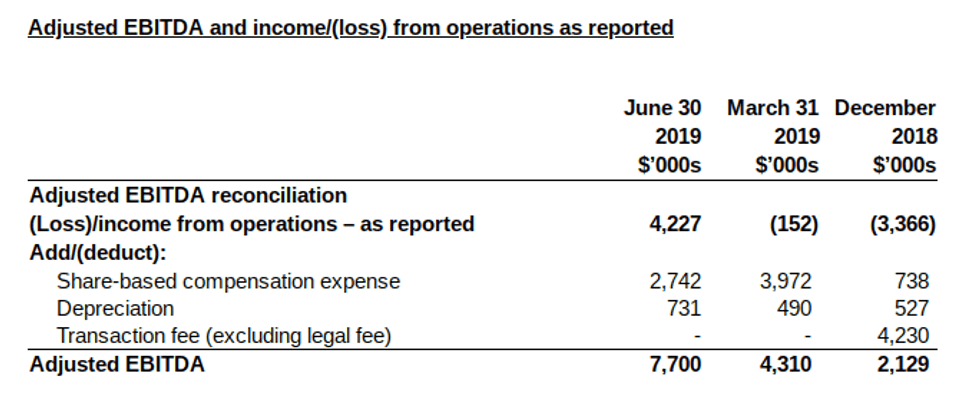
Adjusted EBITDA is not a recognized performance measure under IFRS, does not have a standardized meaning and therefore may not be comparable to similar measures presented by other issuers. Adjusted EBITDA is included as a supplemental disclosure because Management believes that such measurement provides a better assessment of the Company’s operations on a continuing basis by eliminating certain non-cash charges and charges or gains that are nonrecurring. Adjusted EBITDA is defined as net loss excluding interest, taxes, depreciation and amortization, and share-based compensation. Adjusted EBITDA has limitations as an analytical tool as it does not include depreciation and amortization expense, interest income and expense, taxes, share-based compensation and transaction fees. Because of these limitations, Adjusted EBITDA should not be considered as the sole measure of the Company’s performance and should not be considered in isolation from, or as a substitute for, analysis of the Company’s results as reported under IFRS. The most directly comparable measure to Adjusted EBITDA calculated in accordance with IFRS is operating income (loss). The above is a reconciliation of the Company’s operating loss to Adjusted EBITDA. See “Reconciliation of non-IFRS measures” in the Company’s Management’s Discussion and Analysis for the three and six-month periods ended June 30, 2019 for additional information.
For more information:
Laura Lepore, VP, Investor Relations
Telephone: 705-719-7425 ext 216
Email: investors@medipharmlabs.com
Website: www.medipharmlabs.com
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION:
This news release contains “forward-looking information” and “forward-looking statements” (collectively, “forward-looking statements”) within the meaning of the applicable Canadian securities legislation. All statements, other than statements of historical fact, are forward-looking statements and are based on expectations, estimates and projections as at the date of this news release. Any statement that involves discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions, future events or performance (often but not always using phrases such as “expects”, or “does not expect”, “is expected”, “anticipates” or “does not anticipate”, “plans”, “budget”, “scheduled”, “forecasts”, “estimates”, “believes” or “intends” or variations of such words and phrases or stating that certain actions, events or results “may” or “could”, “would”, “might” or “will” be taken to occur or be achieved) are not statements of historical fact and may be forward-looking statements. In this news release, forward-looking statements relate to, among other things, ramping up of production capacity, a growing customer base, capacity increases and timing thereof, commencing shipping to additional provincial distributors, expanded licensed space and timing thereof, new product manufacturing, expected processing and sales in Q3 2019, Soft Gel capabilities and timing thereof, the manufacturing and supply of vapourizer cartridges and timing thereof, future cannabis product innovation, growing demand for specialty concentrate based consumer end products, broadening global distribution, completion of MediPharm Labs Australia’s facility and timing thereof, and the expected processing capacity of the Australian facility. Forward-looking statements are necessarily based upon a number of estimates and assumptions that, while considered reasonable, are subject to known and unknown risks, uncertainties, and other factors which may cause the actual results and future events to differ materially from those expressed or implied by such forward-looking statements. Such factors include, but are not limited to: general business, economic, competitive, political and social uncertainties; the inability of MediPharm Labs to obtain adequate financing; the delay or failure to receive regulatory approvals; and other factors discussed in MediPharm Labs’ filings, available on the SEDAR website at www.sedar.com. There can be no assurance that such statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on the forward-looking statements and information contained in this news release. Except as required by law, MediPharm Labs assumes no obligation to update the forward-looking statements of beliefs, opinions, projections, or other factors, should they change.
Click here to connect with MediPharm Labs Corp. (TSX:LABS) for an Investor Presentation.
Source: www.globenewswire.com