Avisa Diagnostics: Developing A Thermometer for the Lungs
Avisa Diagnostics (CSE:AVBT) has launched its campaign on the Investing News Network
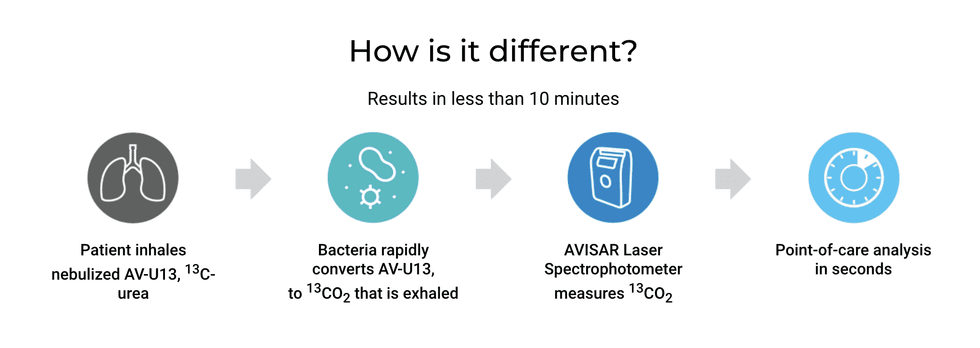
Avisa Diagnostics (CSE:AVBT) specializes in respiratory disease diagnostics through its rapid, point-of-care Avisa BreathTest solution™. This simple, ultra-rapid breath test was designed to save time, money and lives, and has been dubbed a “thermometer for the lungs. ” The compelling new technology measures bacterial load in the lungs enabling better diagnoses, monitor therapy, and helping to mitigate the overuse of broad-spectrum antibiotics.
The Avisa BreathTest™ is a novel drug/device biomarker technology platform that enables the ultra-rapid detection of virulent bacterial pathogens. It works by detecting and monitoring bacterial load after the patient inhales or ingests its drug substrates via proprietary delivery systems.
Avisa has established clinical proof-of-concept through trials in cystic fibrosis, tuberculosis and community-acquired pneumonia, which demonstrated positive safety and clinical efficacy results.
Avisa Diagnostics’ Company Highlights
- Avisa Diagnostics is a clinical-stage medical device company developing the Avisa BreathTest™, a novel drug/device biomarker technology platform that enables the ultra-rapid detection of virulent bacterial pathogens, detecting and monitoring bacterial load after the patient inhales or ingests its drug substrates via proprietary delivery systems.
- The respiratory disease diagnostics market alone is expected to be worth $6.7 billion by 2025, and the Avisa BreathTest™ is a unique technology addressing a prevalent issue within this growing market.
- 1.7 million people are on ventilators in the US alone, and nearly half of all individuals on a ventilator get ventilator-associated pneumonia, a potentially fatal condition. The Avisa breath test allows for unprecedented monitoring and has the potential to save thousands of lives.
- Avisa is currently in the process of putting together their investigational new device exemption for the FDA. Pending approval, they will then be able to move into commercialization.
- Avisa’s management team has decades of combined experience in the development and commercialization of medical technology, diagnostics and clinical regulation and strategy. Experience in these key industries put them in a strong position to move the Avisa BreathTest™ through clinical trials and into commercialization.