Botanix Relishes Scheduling Update for Synthetic CBD
Botanix Pharmaceuticals announced a critical scheduling update from the US Drug Enforcement Administration regarding synthetic CBD used by its business associate Purisys.
Botanix Pharmaceuticals (ASX:BOT) noticed a boost thanks to a regulation change for the synthetic cannabidiol (CBD) produced with its business partner, Purisys.
In a press release issued on Monday (November 25), the company said that on November 22 Purisys received confirmation that its CBD, which carries less than 0.001 percent of tetrahydrocannabinol (THC), was removed from the scheduling as a controlled substance by the US Drug Enforcement Administration (DEA) within the Controlled Substances Act.
“This change in the regulation of synthetic CBD in the US will make a major difference to the speed of developing Botanix products and greatly reduces the risks and costs of clinical development,” Vince Ippolito, executive chairman and president of Botanix, said in a press release.
The executive said this ruling from the American federal authority comes at just the right time for the company since it’s getting ready for studies in its dermatology programs.
Botanix and Purisys are tied together by way of a supply agreement signed in October.
The duo is celebrating the change from the DEA; prior to this resolution, the use of synthetic CBD for clinical studies needed licensing from the federal agency. According to Botanix, this led to “significant management and cost overheads to Botanix’s pharmaceutical development activities.”
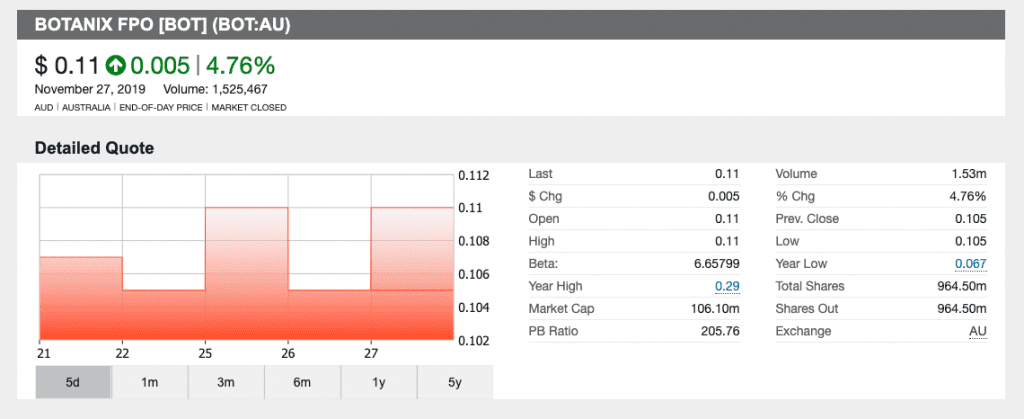
Shares of Botanix jumped nearly 5 percent on Monday when it first informed investors of the crucial change. As of Wednesday’s (November 27) trading session, the company is up nearly 5 percent for a price per share of AU$0.11.
Don’t forget to follow us @INN_Australia for real-time news updates!
Securities Disclosure: I, Bryan Mc Govern, hold no direct investment interest in any company mentioned in this article.
