Metamaterials Enabling Medical Breakthroughs, Promise Affordable, Accurate Point-of-Care Diagnostics - Part 1
Part one of this series covers the long-term trend toward point-of-care technologies and companies involved in diabetes management.
Dr. McCoy’s medical tricorder from the iconic Star Trek series is an ideal which medical researchers and device developers continue to pursue: a non-invasive, point-of-care, diagnostic tool, able to see inside the human body and make an immediate diagnosis. However, a fundamental challenge is that the skin reflects electromagnetic waves and prevents incoming signals from entering the body.
Metamaterial Inc. (CSE:MMAT) has developed metamaterial films which cancel skin reflections, increasing the signal to noise ratio. The Company is developing several applications which integrate this technology, including pain-free, non-invasive blood glucose monitoring for diabetics; earlier breast cancer screening; higher quality, lower cost MRI imaging; and more rapid treatment of stroke.
This article is the first of a three part series. Part 1 covers the long-term trend toward point-of-care (POC) technologies and companies involved in diabetes management. Part 2 (still to come) examines medical imaging including mammography and MRI, and Part 3 (still to come) explores traumatic brain injury (TBI), concussion and stroke.
Point-of-care diagnostics (POCDs) are diagnostic tests that can be administered anywhere that a patient or consumer is receiving care. POCDs have the potential to increase the efficacy of treatments, as medical professionals are informed on the spot about a patient’s condition and can quickly administer the solutions. POCDs are typically used to predict or monitor biomarkers in chronic conditions like diabetes, cardiovascular diseases (CVD) and respiratory diseases. Some prominent examples are glucose strips that use blood samples to monitor glucose levels of diabetes patients, EKG devices for cardiovascular diseases and spirometers for asthma.
Today, modern diagnostic devices are helping to decentralize the medical industry through portable and efficient devices that allow patients to safely administer their own tests and adjust treatment accordingly. For example, pulse oximetry devices can test arterial oxygen saturation in a quick, simple, non-invasive and affordable way. Previously, an intra-arterial needle puncture was required before the results were sent to a laboratory for testing. Rapid diagnostic tests like malaria antigen detection tests rely on a state of the art in immunology that did not exist until recent decades.
“POC technologies have the potential to improve the management of various diseases and conditions, and these devices can be simple enough to be used at the primary care level and in remote settings with no laboratory infrastructure,” said Milos Todorovic, Lux Research Analyst. “Nonetheless, the home care market will take up to a decade to mature, depending on disease application. Meanwhile, care settings like the doctor’s office will see faster adoption of POC solutions.”
Diabetes and point-of-care diagnostics
Diabetes affects how the body uses glucose, the principal type of blood sugar. A person’s blood glucose level rises after eating as the body breaks down food, which causes the pancreas to secrete a hormone, insulin, into the blood. Insulin allows glucose to be absorbed into the body’s cells to be used for energy.
In patients with Type 1 diabetes, which accounts for around 5 percent of diagnosed cases in the U.S., the pancreas does not make enough insulin to regulate blood glucose. In the most common form of diabetes, Type 2, the body becomes resistant to insulin, and the pancreas may not be able to supply enough to regulate blood glucose. Type 1 patients are often recommended to measure blood glucose up to 10 times per day. Type 2 diabetics who take insulin may also need to test their blood sugar level several times a day.
Forty years ago, the first routinely available home glucose measurement kits were introduced. These new tools helped to greatly decentralize diabetes management, saving many trips to the hospital or clinic. However, they were large devices and required painful daily or even more frequent needle sticks to test the blood. The devices today are small, portable and easy to use. But the process remains cumbersome and painful, requires multiple needle sticks and test strips are costly over time. Due to the widespread nature of diabetes, decentralized diagnostic solutions have the potential to benefit millions of patients.
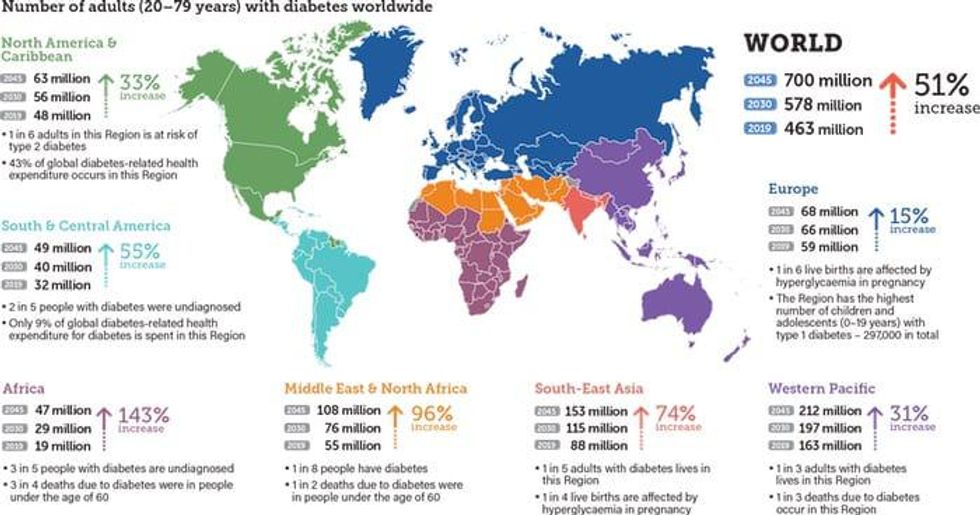
According to recent estimates from the IDF Diabetes Atlas, approximately 463 million people in the world were living with diabetes in 2019, which is nearly a 400 percent increase since 1980. According to Grand View Research, the glucose monitoring device market is projected to grow to reach US$10.4 billion by 2027 at a compound annual growth rate (CAGR) of 12.7 percent.
The global prevalence of diabetes has drawn the attention of leading medical technology companies and researchers that are working to develop a non-invasive solution capable of accurately detecting glucose levels without pain. To some in the medical community, non-invasive glucose monitoring has become the holy grail of medical diagnostics, which has helped drive research and development in the sector.
Leading blood glucose monitoring solutions
There are a number of innovative technology and medical device companies working to service diabetes patients around the world through decentralized solutions, including global leaders such as Abbott (NYSE:ABT) that are working to develop POCDs with the potential to improve the medical diagnostic and testing process. The company is developing a blood analyzer capable of offering a variety of blood diagnostic tests on a single point-of-care platform. According to Ulf Martin Schilling MD Ph.D. at the Center of Clinical and Experimental Medicine, University of Linköping, the deployment of point of care devices could save between 8-20 percent of laboratory costs through shortened turnaround times and improving decision-making processes.
Continuous glucose monitoring (CGM) devices have also emerged to provide real-time information to patients that need to keep a close watch over their blood levels. According to Diabetes Canada, CGM devices have been shown to benefit both adults and children with type 1 diabetes who have not achieved their blood sugar targets or have difficulty with hypoglycemia. While CGM systems can be expensive compared to other glucose monitoring methods, the real-time data can provide key insights for patients including glucose rates, the rate of change and the direction is moving throughout the day.
Continuous glucose monitoring expert Dexcom (NASDAQ:DXCM) has designed a proprietary CGM system, the DexCom G6, to offer the full benefits of CGM technology including continuous data visibility and real-time monitoring. The DexCom G6 system comes loaded with a compatible Follow App that allows family members and caretakers to remotely monitor blood levels, providing patients in need with additional peace of mind. Like many CGM systems, the G6 does not require fingersticks, allowing patients to monitor blood levels pain-free without the need for calibration.
Metamaterial Inc. is developing GlucoWise®, a wireless sensing device which measures blood glucose non-invasively. The device gently squeezes the web of skin between the thumb and finger or the earlobe. These areas have adequate blood supply and are thin enough for the signal to pass through the tissue. A signal in the 40-60 GHz range is transmitted through the blood by two sensors with integrated micro-composite metamaterial films, which temporarily make the skin transparent to the radio waves when a measurement is initiated. The amount of glucose changes the dielectric constant of the blood, which in turn affects the transmitted signal.
Metamaterial Inc. has published a pair of peer-reviewed studies in order to assess the performance of its patent-pending technology in controlled pre-clinical environments. The studies were conducted using both human test subjects and animal testing.
The planned GlucoWise® platform encompasses a pain-free, non-invasive sensor, a companion mobile app for smart devices, and smart cloud-based personalized advice and alerts. Additionally, GlucoWise® does not require the use of consumables.
Pre-clinical studies using GlucoWise® prototypes have shown promising early results. Metamaterial Inc. remains focused on developing its prototypes in order to deliver a finished commercial product in the future. A series of clinical trials with larger numbers of participants will be needed to verify performance across patient variables including age, gender, ethnicity, skin type and coloration. META is working to improve specificity, sensitivity and robustness, to integrate discrete electronic components and miniaturize the device. The company is seeking a strategic OEM partner to assist with these efforts.
Takeaway
There are millions of people around the world suffering from diabetes and other major medical conditions that can be diagnosed and monitored using medical devices capable of detecting blood levels. As improvements in medical technology help to enhance the capabilities of patients, all levels of the medical industry stand to benefit as savings in resources are passed down to clinics and hospitals. Innovations in metamaterials and sensor technology have shown the potential to greatly enhance medical devices, driving the decentralization of healthcare in the process.
This INNSpired article is sponsored by Metamaterial Inc. (CSE:MMAT). This INNSpired article provides information that was sourced by the Investing News Network (INN) and approved by Metamaterial Inc. in order to help investors learn more about the company. Metamaterial Inc. is a client of INN. The company’s campaign fees pay for INN to create and update this INNSpired article.
INN does not provide investment advice and the information on this profile should not be considered a recommendation to buy or sell any security. INN does not endorse or recommend the business, products, services, or securities of any company profiled.
The information contained here is for information purposes only and is not to be construed as an offer or solicitation for the sale or purchase of securities. Readers should conduct their own research for all information publicly available concerning the company. Prior to making any investment decision, it is recommended that readers consult directly with Metamaterial Inc. and seek advice from a qualified investment advisor.



