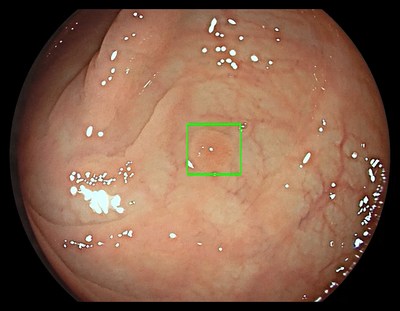
- The GI Genius ™ Module Assists Physicians in Detecting Pre-Cancerous Growths; Potentially Addressing 19 Million Colonoscopies Annually
- Transformative AI Colorectal Cancer Prevention Platform Expands Medtronic Capabilities in Medical Technology Data Analytics and Insights
Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced the U.S. Food and Drug Administration (FDA) granted de novo clearance for GI Genius™ intelligent endoscopy module in the United States . The GI Genius module is the first and only commercially available computer-aided detection (CADe) system using artificial intelligence (AI) to identify colorectal polyps. The module, compatible with any colonoscope video, provides physicians with a powerful new solution in the fight against colorectal cancer — the third most common form of cancer globally with 1.8 million new cases in 2018. 1
"More than 19 million screening colonoscopies are performed in the United States each year. A key factor in the prevention of colorectal cancer is the integration of leading-edge technologies into gastroenterology practices to increase detection rates," said Dr. James Weber , a gastroenterologist and chief executive officer of the GI Alliance. "Detection of adenomas during colonoscopy is an important quality metric. The addition of AI can increase the quality of colonoscopies, potentially improving diagnosis and outcomes for colon cancer patients."
"Colonoscopies allow highly skilled gastroenterologists to identify polyps and lesions that might develop into cancer. With GI Genius we can tap into the potential of artificial intelligence approaches to increase detection rates. This important new development helps us in our mission to detect colon cancer early and to improve patient outcomes," said Michael Sapienza , chief executive officer of the Colorectal Cancer Alliance.
The GI Genius module uses advanced AI to highlight the presence of precancerous lesions with a visual marker in real-time – serving as an ever vigilant second observer. It processes images using advanced algorithms that can identify and mark abnormalities consistent with polyps, including small flat polyps that might otherwise go undetected by the human eye. Studies have shown that having a second observer can increase polyp detection rates and every 1% increase in adenoma detection rate (ADR) reduces the risk of colorectal cancer by 3%. 2,3 Use of the GI Genius module has demonstrated a 14% absolute increase in ADR compared to colonoscopy alone for both flat (42% increase) and polyploid (36% increase) lesions, thus increasing accuracy and reducing the rise of interval cancers which can occur between colonoscopies. 4
"Medtronic is committed to preventing colorectal cancer and improving patient outcomes with disruptive technologies that aid screening, increase patient compliance, and improve treatment," said Giovanni Di Napoli , president of the Gastrointestinal business, which is part of the Medical Surgical Portfolio at Medtronic. "With FDA de novo clearance for the GI Genius and its AI capabilities, we expect to enhance and improve colonoscopies and polyp detection. By introducing AI technology into the colonoscopy market, we anticipate improving colonoscopy detection rates and reducing variability in patient outcomes."
Medtronic is the exclusive worldwide distributor of the GI Genius module, which was developed and is manufactured by Cosmo Pharmaceuticals. The GI Genius intelligent endoscopy module received de novo clearance from the U.S. FDA on April 9, 2021 . In addition to the United States , the GI Genius module is available in Europe and select markets in Asia , Australia and the Middle East .
About Medtronic
Medtronic plc ( www.medtronic.com ), headquartered in Dublin, Ireland , is among the world's largest medical technology, services and solutions companies – alleviating pain, restoring health and extending life for millions of people around the world. Medtronic employs more than 90,000 people worldwide, serving physicians, hospitals and patients in more than 150 countries. The company is focused on collaborating with stakeholders around the world to take healthcare Further, Together.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
For detailed information regarding the instructions for use, indications, contraindications, warnings, precautions, and potential adverse events, please consult the device manual. For further information, contact your local Medtronic representative.
- Globocan. Estimated number of deaths in 2018 worldwide, both sexes, all ages. Globocan Cancer Data. Published 2018. Accessed Jan 2021 .
- Wang P, Berzin TM, Glissen Brown JR , et al. Gut 2019; 68:1813-1819.
- Corley DA, Jenson CD, Marks AR JR, et al. NEJM 2014;370:1298-306.
- Repici, A., Badalamenti, M., Maselli, R., et al. Gastroenterology 2020; doi: https://doi.org/10.1053/j.gastro.2020.04.062 .
| Contacts: | |
| John Jordan | Ryan Weispfenning |
| Public Relations | Investor Relations |
| +1-508-452-4891 | +1-763-505-4626 |
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/us-fda-grants-de-novo-clearance-for-first-and-only-artificial-intelligence-system-for-colonoscopy-medtronic-launches-gi-genius-intelligent-endoscopy-module-301266276.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/us-fda-grants-de-novo-clearance-for-first-and-only-artificial-intelligence-system-for-colonoscopy-medtronic-launches-gi-genius-intelligent-endoscopy-module-301266276.html
SOURCE Medtronic plc

![]() View original content to download multimedia: https://www.newswire.ca/en/releases/archive/April2021/12/c2450.html
View original content to download multimedia: https://www.newswire.ca/en/releases/archive/April2021/12/c2450.html