Salix Pharmaceuticals Releases Fourth Annual Patient Perspectives IBS Impact Report
Bausch Health Companies Inc. (NYSE:BHC)(TSX:BHC) and its gastroenterology (GI) business, Salix Pharmaceuticals ("Salix"), today for IBS Awareness Month announced the results from the fourth edition of its annual survey of U.S adults living with irritable bowel syndrome (IBS) or chronic idiopathic constipation (CIC). Developed as a nationwide survey conducted in partnership with Fairleigh Dickinson University Poll (FDU Poll), more than 850 IBSCIC patients were surveyed to better understand their experiences. The findings illustrate the current behaviors and experiences of the IBS and CIC patient population
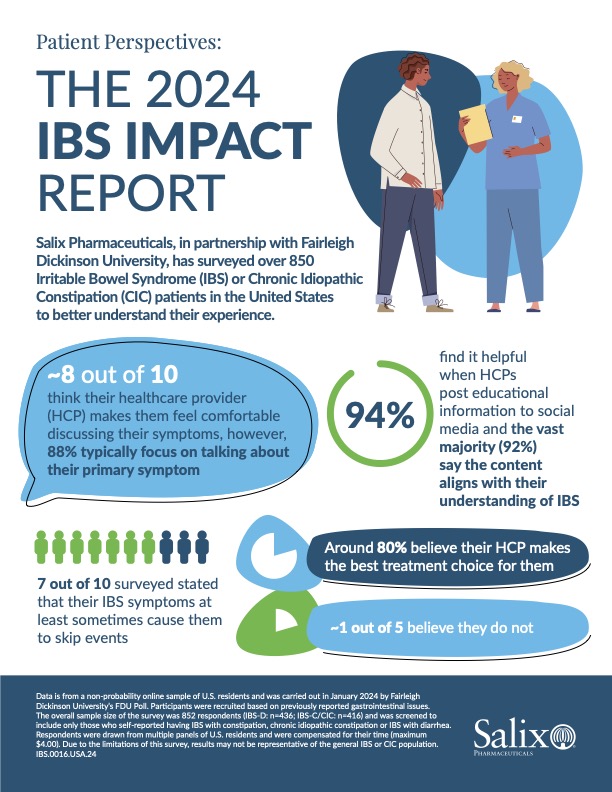
Notably, more than half of IBS/CIC patients surveyed said they feel alone in their experience and try to hide symptoms from family and friends. Additionally, 7 out of 10 respondents stated that their IBS symptoms cause them to skip events. Patients went on to reveal not only do they struggle to talk to their doctor about all of their symptoms, but also that their treatments often do not address the totality of those symptoms. The report unveiled trends related to social media too, suggesting it may bolster a sense of community and inspire more authentic conversations with their healthcare provider.

"For 35 years, we've been working to make a difference in the lives of millions of Americans living with GI disease," shares Nicola Kayel, Senior Vice President, Marketing, Salix. "Our fourth annual IBS Impact Report underscores our steadfast commitment to improving patient lives. The findings of this year's survey highlight the need for more authentic conversation around IBS experiences and impact on daily living. We are confident the findings will encourage more productive dialogue between healthcare providers and their patients, and also across social media platforms where patients can truly foster community."
Patient advocacy group, the International Foundation for Gastrointestinal Disorders (IFFGD), has been at the forefront of these patient conversations since its founding in 1991. President of IFFGD, Ceciel T. Rooker, stated "At IFFGD, we are committed to broadening patient understanding about GI disorders, like IBS, and insights such as those from the 2024 IBS Impact Report by Salix help us raise visibility of the patient experience and validate what we hear from patients firsthand. IBS symptoms change over time and are hard to talk about. The fact that most patients aren't addressing all of their symptoms with their healthcare provider emphasizes that there is work to be done to reduce stigma around this condition. We recommend patients find a healthcare provider that they feel comfortable being open with and that will work with them long-term to manage and treat their IBS."
Additional findings include:
- 94% of respondents find it helpful when healthcare providers post to social media and the vast majority (92%) say the content of these posts aligns with their understanding of IBS
- Over 90% of IBS respondents find it informative when people post on social media about what they're going through; seeing posts about IBS helps to normalize it
- 8 out of 10 respondents think their healthcare providers make them feel comfortable discussing their symptoms, however, 88% typically focus on talking about their primary symptom
- Approximately 8 out of 10 surveyed believe their healthcare providers make the best treatment choice for them, while about 2 out of 10 believe they do not
- More specifically:
- 96% of IBS-C/CIC respondents want their healthcare provider to understand all the symptoms they are experiencing rather than just the primary one
- More than three-quarters of IBS-C/CIC patients report their current treatment is not meeting all treatment goals, citing the top 3 unresolved symptoms as bloating, abdominal discomfort and stomach pain
- 95% of IBS-D respondents want their healthcare providers to ask about how their symptoms impact daily living
- Roughly 3 in 4 IBS-D respondents report their current treatment was not meeting all treatment goals citing the top 2 unresolved symptoms as diarrhea and stomach pain
###
About the Methodology
The survey was conducted online in January 2024 in the U.S. by Fairleigh Dickinson University Polls on behalf of Salix Pharmaceuticals among 852 U.S. residents aged 18+, including 416 who have been diagnosed with IBS-C or CIC, and 436 who have been diagnosed with IBS-D. Respondents for this survey were selected from among those who have agreed to participate in our surveys. Respondents were compensated for their time up to $4.00. Due to the limitations of this survey, results may not be representative of everyone in the U.S. who have been diagnosed with IBS-C, CIC and/or IBS-D.
About FDU Poll
Since 2001, FDU Poll (a division of Fairleigh Dickinson University) has conducted survey research on issues of public importance. Utilizing best practices in survey methodology, the Poll produces research that is conducted nationally and statewide. Findings from FDU's surveys have been reported on numerous regional, national and international media outlets such as the New York Times, Washington Post, Wall Street Journal, Star-Ledger, as well as local and national broadcast media outlets. The FDU Poll is in the top tier of polls nationwide. Poll aggregator Five Thirty-Eight has released its new rankings of polls ranking FDU 31st in the country, out of more than 500.
About Salix
Salix Pharmaceuticals is one of the largest specialty pharmaceutical companies in the world committed to the prevention and treatment of gastrointestinal diseases. For more than 30 years, Salix has licensed, developed, and marketed innovative products to improve patients' lives and provide health care providers with life-changing solutions for many chronic and debilitating conditions. Salix currently markets its product line to U.S. health care providers through an expanded sales force that focuses on gastroenterology, hepatology, pain specialists, and primary care. Salix is headquartered in Bridgewater, New Jersey. For more information about Salix, visit www.Salix.com and connect with us on Twitter and LinkedIn.
About Bausch Health
Bausch Health Companies Inc. (NYSE:BHC)(TSX:BHC) is a global diversified pharmaceutical company enriching lives through our relentless drive to deliver better health outcomes. We develop, manufacture and market a range of products, primarily in gastroenterology, hepatology, neurology, dermatology, medical aesthetic devices, international pharmaceuticals, and eye health, through our controlling interest in Bausch + Lomb. Our ambition is to be a globally integrated healthcare company, trusted and valued by patients, HCPs, employees and investors. For more information, visit www.bauschhealth.com and connect with us on Twitter and LinkedIn.
| Investor Contact: | Media Contact: | |
| ir@bauschhealth.com | Kevin Wiggins | |
| (877) 281-6642 (toll-free) | corporate.communications@bauschhealth.com | |
| (908) 541-3785 | ||
| Gianna Scalera | ||
| salixcommunications@bauschhealth.com | ||
| (908) 541-2110 |
SOURCE: Bausch Health Companies Inc.
View the original press release on accesswire.com





