bbott (NYSE: ABT) today announced the U.S. launch of NeuroSphere™ Virtual Clinic, a first-of-its-kind technology that allows patients to communicate with physicians, ensure proper settings and functionality, and receive new treatment settings remotely as needed. Approved by the U.S. Food and Drug Administration, the NeuroSphere Virtual Clinic has the potential to increase access to optimal treatment for patients suffering from chronic pain or movement disorders who don't live close to a care provider, have difficulty accessing care, or are unable to go to the doctor because of circumstances like COVID-19.
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8828751-abbott-introduces-neurosphere-virtual-clinic/
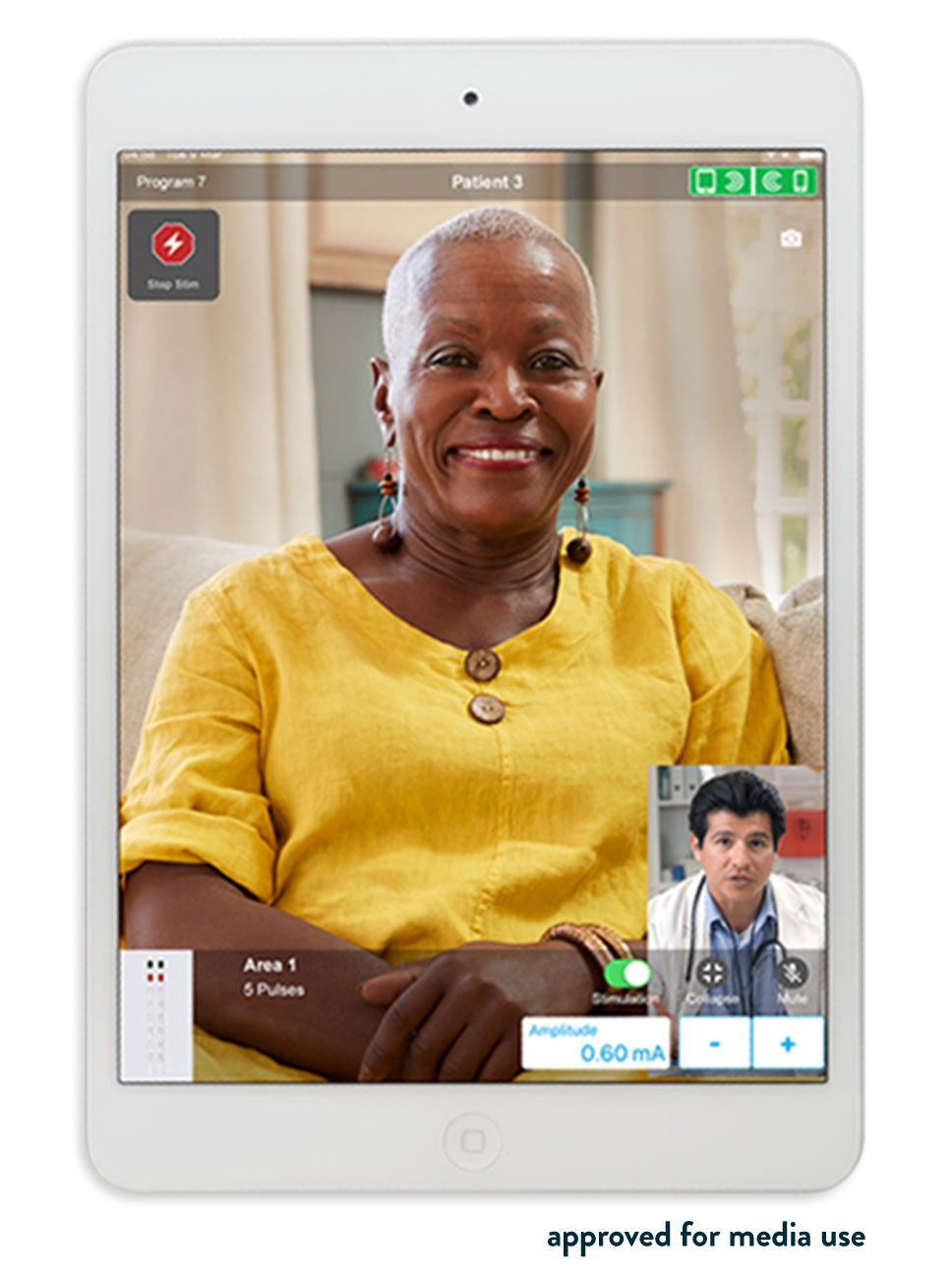
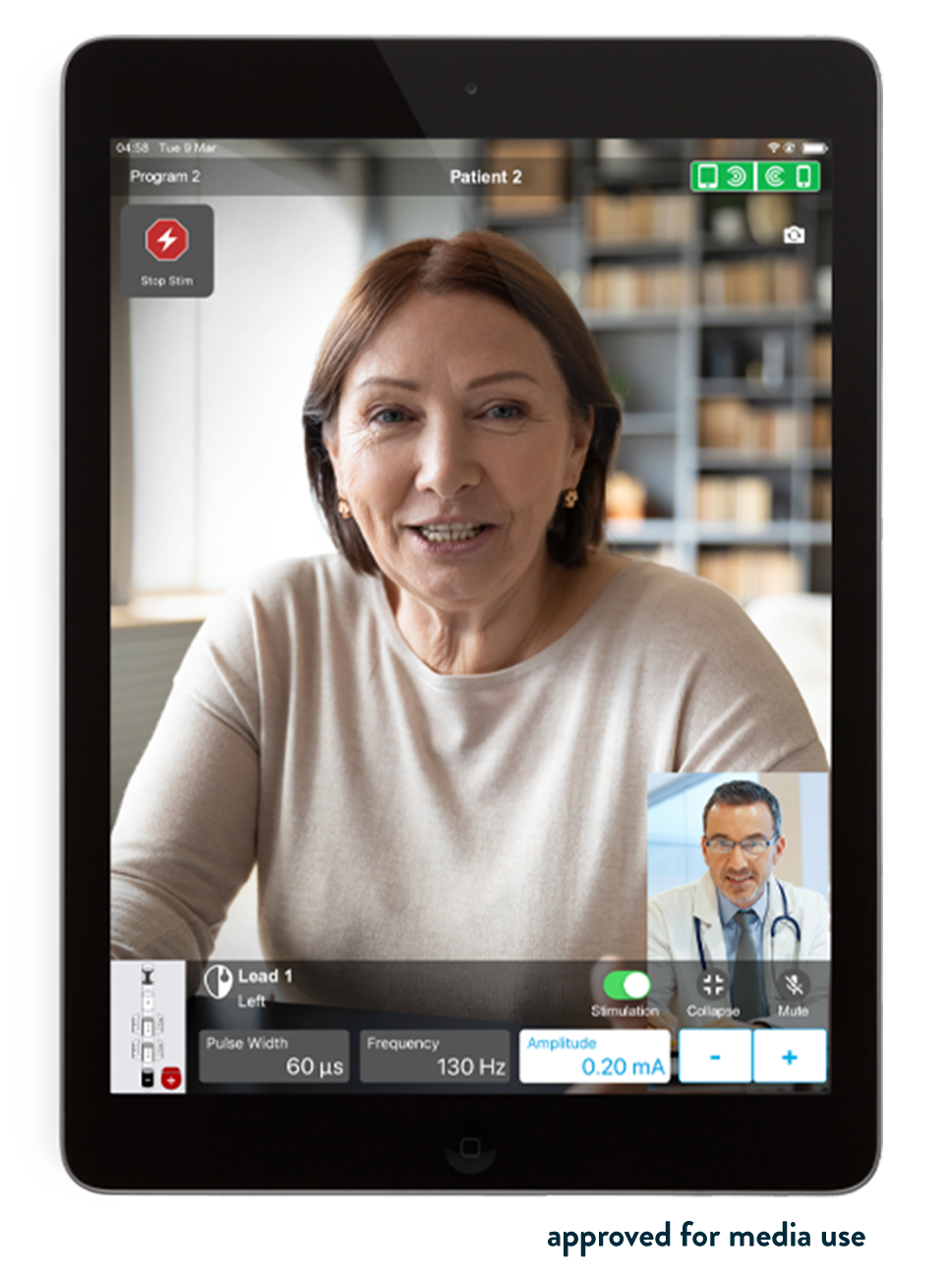
Abbott's NeuroSphere Virtual Clinic gives patients the flexibility and comfort of receiving care anywhere* by connecting with their doctor via secure in-app video chat and an integrated remote programming feature, now available within the proprietary Abbott patient controller app. NeuroSphere Virtual Clinic enables clinicians to prescribe new treatment settings remotely to the patient's neurostimulation device using the clinician programmer app and a new, simple and secure remote care connection. This advancement gives patients confidence in their care and the convenience to manage their therapy in a way that fits their lifestyle.
"With NeuroSphere Virtual Clinic, physicians can communicate and digitally prescribe new stimulation settings remotely, allowing them to extend care beyond their clinic walls and optimize therapy management," said Timothy Deer , M.D., DABPM, president and chief executive officer of The Spine and Nerve Center of the Virginias in Charleston, W.Va. "This is a significant advancement for chronic pain patients."
"NeuroSphere Virtual Clinic solves considerable issues patients with movement disorders, such as Parkinson's disease or essential tremor, can have in obtaining the care they need," said Drew Falconer , M.D., neurologist and director, Inova Parkinson's and Movement Disorders Center in Fairfax, Va. "Often, patients must be off their medication overnight, so that their treatments can be adjusted properly, which can make it difficult for a patient to travel to their specialist. With NeuroSphere Virtual Clinic, patients can receive stimulation settings from their physicians in real time and remotely via cloud and Bluetooth-based technology, which is something we have never been able to do before. This opens up a world in which patients can receive the care they need anytime, anywhere."*
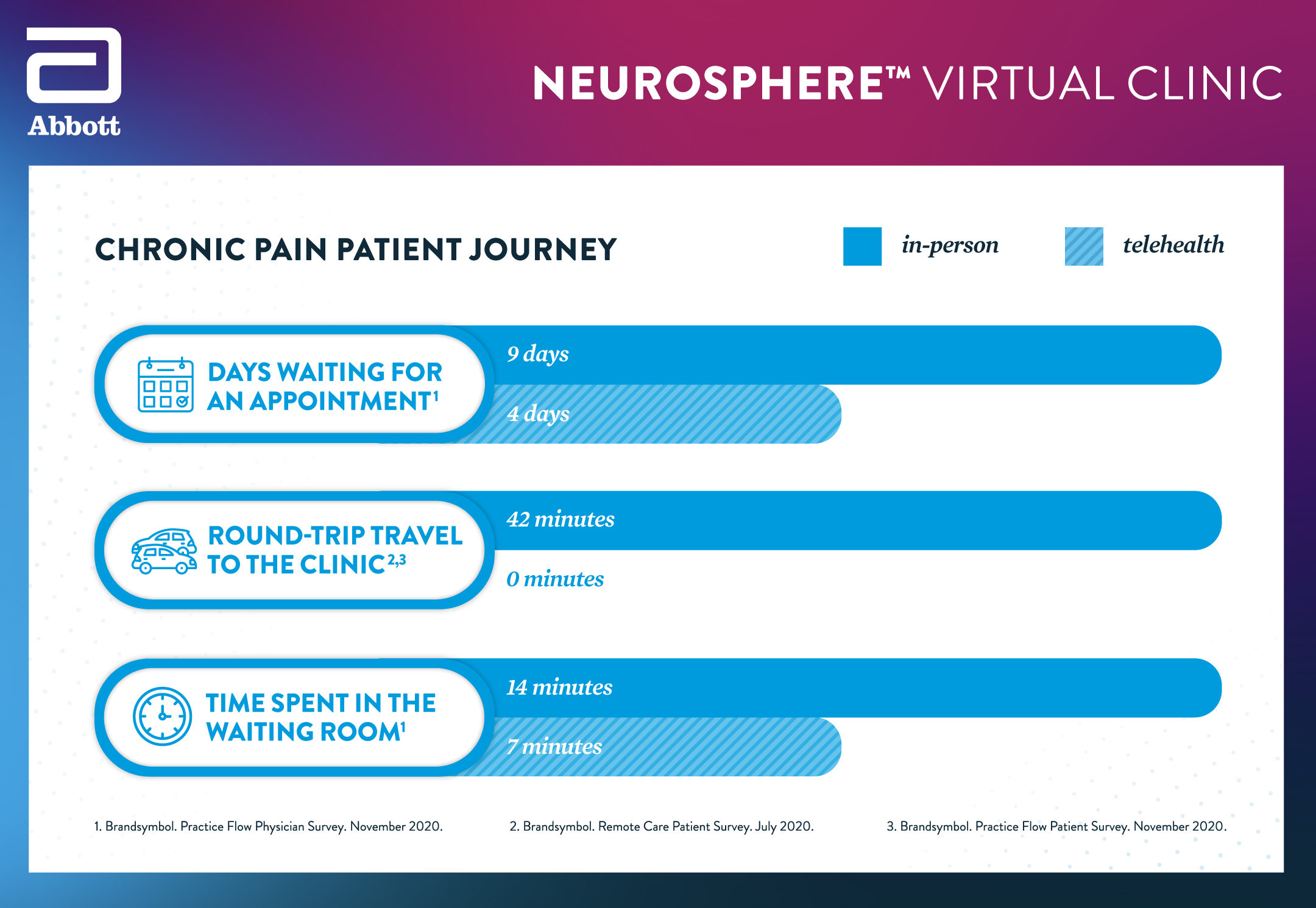
The NeuroSphere Virtual Clinic can also be helpful for people who live in areas — both rural and urban — with inadequate access to medical services. On average people living with movement disorders will travel over 150 miles to access specialists offering deep brain stimulation (DBS). 1 Without alternative solutions, such as digital and/or telehealth options, these patients are more likely to delay or forego much-needed care. 2 NeuroSphere Virtual Clinic brings the convenience and flexibility of telemedicine to neurostimulation therapy, further benefitting chronic pain and movement disorder patients with Abbott devices. Medicare will cover remote programming services as a telehealth benefit through the duration of the public health emergency.
"A decade ago, we started evaluating the hurdles that patients had to overcome to receive neuromodulation treatment, and we have been working ever since to find a better way to connect providers and patients – with the goal of empowering patients to decide how to access the care they need," said Keith Boettiger , vice president, Neuromodulation, Abbott. "We are continuing to make these kinds of investments and working with regulatory authorities to make these telehealth changes permanent, as we believe that patients should be able to receive the care they need, regardless of whether they can make it physically to the doctor's office."
The NeuroSphere Virtual Clinic is compatible with Abbott's suite of neuromodulation technologies, including Infinity™ DBS System for patients with Parkinson's disease and tremors of the upper extremities in adults with essential tremors; Proclaim™ XR SCS System for patients living with chronic pain of the trunk and/or limbs; and Proclaim™ DRG Neurostimulation System for patients with chronic pain in the lower limbs caused by complex regional pain syndrome or causalgia. This integration across all Abbott neuromodulation technologies highlights Abbott's relentless pursuit of patient-centered research and development methodologies that use neuroscience combined with cutting-edge technology to go beyond physical symptom relief to improve the lives of people with neurological disorders.
*Anywhere with a cellular or Wi-Fi connection and sufficiently charged patient controller.
For important safety information please visit the websites for Infinity DBS , Proclaim XR and Proclaim DRG devices.
About Neuromodulation
Neuromodulation is an essential treatment that works by delivering electrical treatment directly to a targeted area to alter nerve activity. Neuromodulation is often recommended for patients who suffer from chronic pain and certain movement disorders, such as Parkinson's disease and essential tremors. Currently, more than 50 million Americans suffer from chronic pain 3 , while almost one million people live with Parkinson's disease 4 and an estimated 7 million people live with an essential tremor 5 .
About Abbott
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 109,000 colleagues serve people in more than 160 countries.
Connect with us at www.abbott.com , on LinkedIn at www.linkedin.com/company/abbott-/ , on Facebook at https://www.facebook.com/Abbott and on Twitter @AbbottNews .
1 Abbott Data on File.
2 Deloitte. Narrowing the rural-urban health divide. https://www2.deloitte.com/us/en/insights/industry/public-sector/virtual-health-telemedicine-rural-areas.html . Accessed Nov. 8, 2020 .
3 Centers for Disease Control and Prevention. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults – United States , 2016. https://www.cdc.gov/mmwr/volumes/67/wr/mm6736a2.htm . Accessed Oct. 7, 2020 .
4 Parkinson's Foundation. Statistics. https://www.parkinson.org/Understanding-Parkinsons/Statistics . Accessed Nov. 13, 2020 .
5 National Organization for Rare Diseases. Essential Tremor. https://rarediseases.org/rare-diseases/essential-tremor/ . Accessed Nov. 13, 2020 .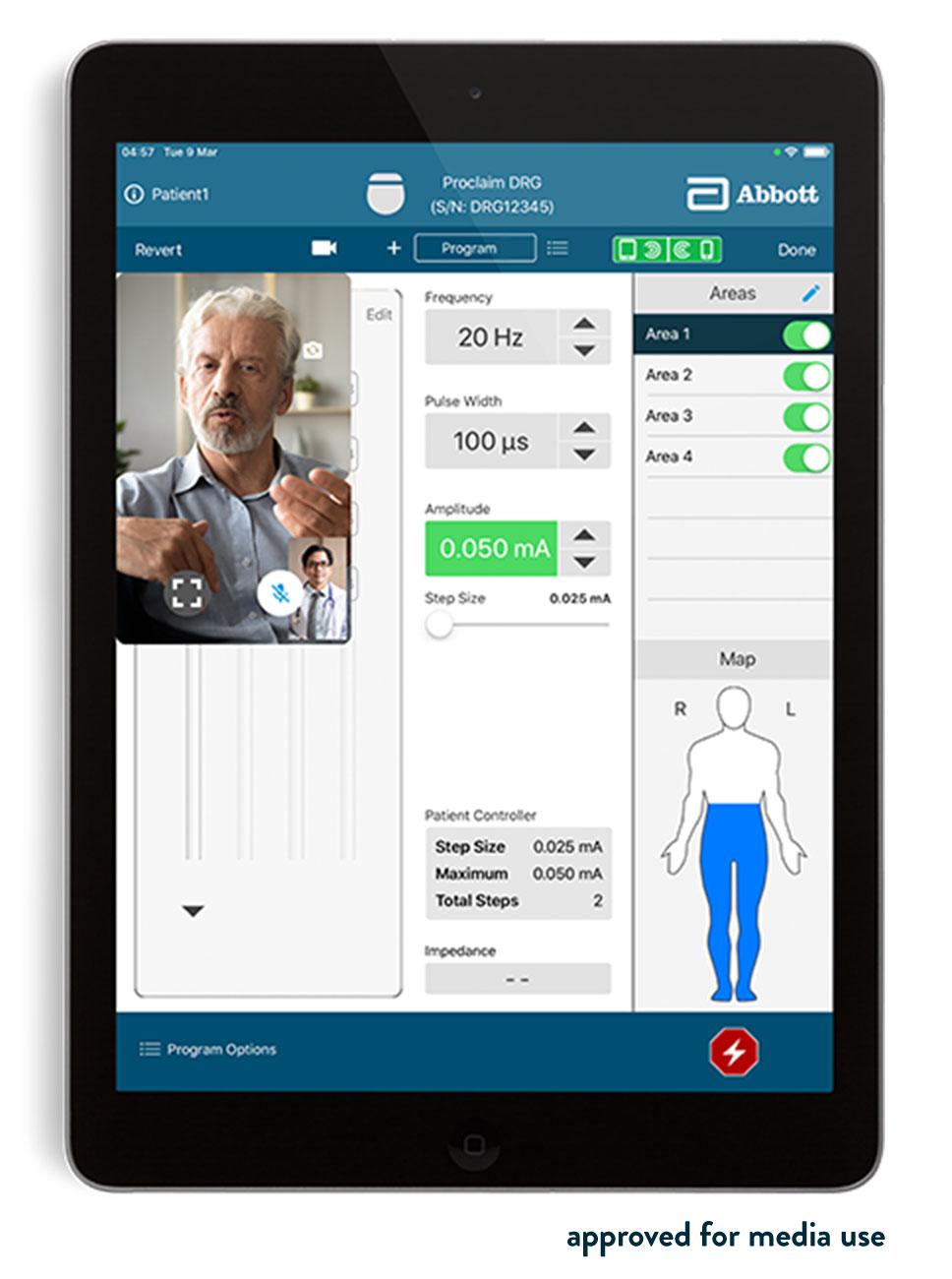


![]() View original content: https://www.prnewswire.com/news-releases/abbott-introduces-neurosphere-virtual-clinic-first-of-its-kind-remote-neuromodulation-patient-care-technology-in-the-us-301241981.html
View original content: https://www.prnewswire.com/news-releases/abbott-introduces-neurosphere-virtual-clinic-first-of-its-kind-remote-neuromodulation-patient-care-technology-in-the-us-301241981.html
SOURCE Abbott








