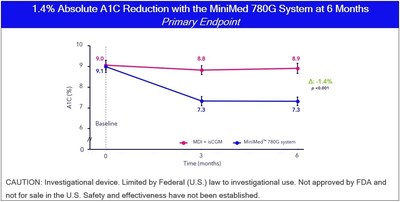
First-of-its-kind study demonstrates individuals using the Medtronic system achieved 1.4% absolute reduction in A1c and 27.6% absolute increase in Time in Range
Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced results from the Medtronic ADAPT study were published in The Lancet Diabetes & Endocrinology . The ADAPT study is the first multi-national randomized controlled study evaluating the performance of MiniMed™ 780G system 1 versus standard of care (multiple daily injections (MDI) + continuous glucose monitoring (CGM)) in individuals with type 1 diabetes not currently meeting glycemic targets. The study evaluated 82 individuals who were using MDI and an intermittently scanned continuous glucose monitor (isCGM) to manage their diabetes prior to trial initiation. On average, individuals enrolled were scanning their isCGM frequently (~9 scansday) yet had suboptimal HbA1C above 8% at baseline.
At study initiation, half of the participants were randomized to stay on standard of care, and the rest transitioned directly to the MiniMed 780G system. Study results showed improvement in glycemic targets for those that transitioned to the MiniMed 780G system with a significant and sustained 1.4% HbA1C reduction at six months. Those using the Medtronic system also saw a 27.6% absolute increase in Time in Range (6.6 more hours/day in target range) compared to those on standard of care without increased in time in hypoglycemia. This improvement was even greater overnight when the algorithm was in full control.
| Time in Range Improvements for Those That Transitioned to the MiniMed 780G System | |||||
| | Standard of Care (MDI + isCGM) | MiniMed 780G System | | ||
| | Baseline | Study | Baseline | Study | Difference |
| Mean Sensor Glucose (SG) | 195.1 mg/dL | 194.7 mg/dL | 208.8 mg/dL | 152.2 mg/dL | -44.9 mg/dL |
| Overall TIR (70 – 180 mg/dL) | 42.6 % | 43.6 % | 36.4 % | 70.6 % | +27.6 % |
| Daytime TIR | - | 43.0 % | - | 68.8 % | +26.2 % |
| Overnight TIR | - | 46.6 % | - | 76.2 % | +30.2 % |
"The ADAPT study illustrates that insulin pump therapy with advanced algorithms, like that of the MiniMed 780G system, can produce significantly improved clinical results versus the current standard of care," said Ohad Cohen , M.D., senior global medical affairs director, Medtronic Diabetes. "Studies like this can change how health care systems define standard of care and expand options for people living with diabetes to begin using insulin pumps sooner to improve their glycemic control and help reduce the burden of diabetes."
When comparing A1C results at 6 months, 27.8% of individuals using the MiniMed 780G system in the study achieved an HbA1c below 7%, while no individuals that remained on MDI +isCGM achieved that desired result.
| Significantly More Participants Achieved Glycemic Targets at 6 Months with the MiniMed 780G System | |||
| | Standard of Care (MDI + isCGM) | MiniMed 780G System | Difference |
| HbA1C | 0 % | 27.8 % | +25.2 % |
| TIR > 70% | 6.5 % | 52.8 % | 51.1 % |
In terms of customer experience, participants using the MiniMed 780G system spent 95.8% of the time in SmartGuard™ (advanced hybrid closed-loop) and experienced few system exits (only 0.9 SmartGuard exits/week. Additionally, the sensor was being used 92.2% of the time (vs. 87.3% in the standard of care group). The ADAPT study also showed that those that transitioned to the MiniMed 780G system experienced a significant increase in treatment satisfaction 2 and reduction in fear of hypoglycemia 3 .
Overall, results showed that the use of the MiniMed 780G system, even when paired with the Guardian™ sensor 3 which requires two fingerstick calibrations per day, had significant improvement across all glycemic metrics compared to standard of care and supports the use at early stages in the treatment pathway given the potential benefits of complication avoidance 4 , treatment satisfaction improvements, and reduced fear of hypoglycemia.
The MiniMed 780G system is now available in over 60 countries around the world and is currently being reviewed by the Food and Drug Administration (FDA) for approval in the U.S.
About the MiniMed 780G system
The MiniMed 780G system is the most advanced insulin pump system from Medtronic, currently approved for the treatment of type 1 diabetes in people aged 7 to 80 years. The MiniMed 780G system's SmartGuard algorithm (also referred to as the advanced hybrid closed-loop algorithm) automates the delivery of insulin every five minutes — personalizing these doses to auto-correct highs every five minutes based on CGM readings 5,6 . The system is designed to be used at a target glucose of 100 mg/dl (5.5 mmol/L) that can be adjusted and personalized on an individual basis.
About the Diabetes Business at Medtronic ( www.medtronicdiabetes.com )
Medtronic is working together with the global community to change the way people manage diabetes. The company aims to transform diabetes care by expanding access, integrating care and improving outcomes, so people living with diabetes can enjoy greater freedom and better health.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland , is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn .
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
1 MiniMed 780G System and Guardian™ 4 sensors are CE Marked only and not commercially available or approved in the U.S.
2 Patient reported outcomes within the ADAPT Study: Diabetes Treatment Satisfaction Questionnaire (DTSQ) and Fear of Hypoglycemia (FHS) Survey
3 Mean DTSQs score for AHCL vs MDI+isCGM arm (6.1 ± 7.55 vs 0.2 ± 6.84, p=0.0003), and HFS scores for AHCL vs MDI+isCGM (-10.2 ± 15.51 vs -2.7 ± 13.08, p = 0.0409)
4 The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin dependent diabetes mellitus NIH external link. New England Journal of Medicine. 1993;329(14):977–986.
5 Carlson, A.L. et al. Safety and glycemic outcomes during the MiniMed™ Advanced Hybrid Closed-Loop system pivotal trial in adolescents and adults with type 1 diabetes. Diab Tech Ther 2021; in press.
6 Collyns.O. et al. Improved Glycemic Outcomes With Medtronic MiniMed Advanced Hybrid Closed-Loop Delivery: Results From a Randomized Crossover Trial Comparing Automated Insulin Delivery With Predictive Low Glucose Suspend in People With Type 1 Diabetes . Diab Care 2021, 44: 969-975
| Contacts: | |
| | |
| Kendra Cassillo | Ryan Weispfenning |
| Public Relations | Investor Relations |
| +1-818-576-5611 | +1-763-505-4626 |
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/medtronic-adapt-study-results-published-in-the-lancet-diabetes--endocrinology-show-improved-glycemic-control-and-treatment-satisfaction-among-those-using-minimed-780g-system-compared-to-insulin-injections-301613854.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/medtronic-adapt-study-results-published-in-the-lancet-diabetes--endocrinology-show-improved-glycemic-control-and-treatment-satisfaction-among-those-using-minimed-780g-system-compared-to-insulin-injections-301613854.html
SOURCE Medtronic plc

![]() View original content to download multimedia: https://www.newswire.ca/en/releases/archive/September2022/01/c9635.html
View original content to download multimedia: https://www.newswire.ca/en/releases/archive/September2022/01/c9635.html