Transformative AI System Enhances Colorectal Cancer Detection Capabilities
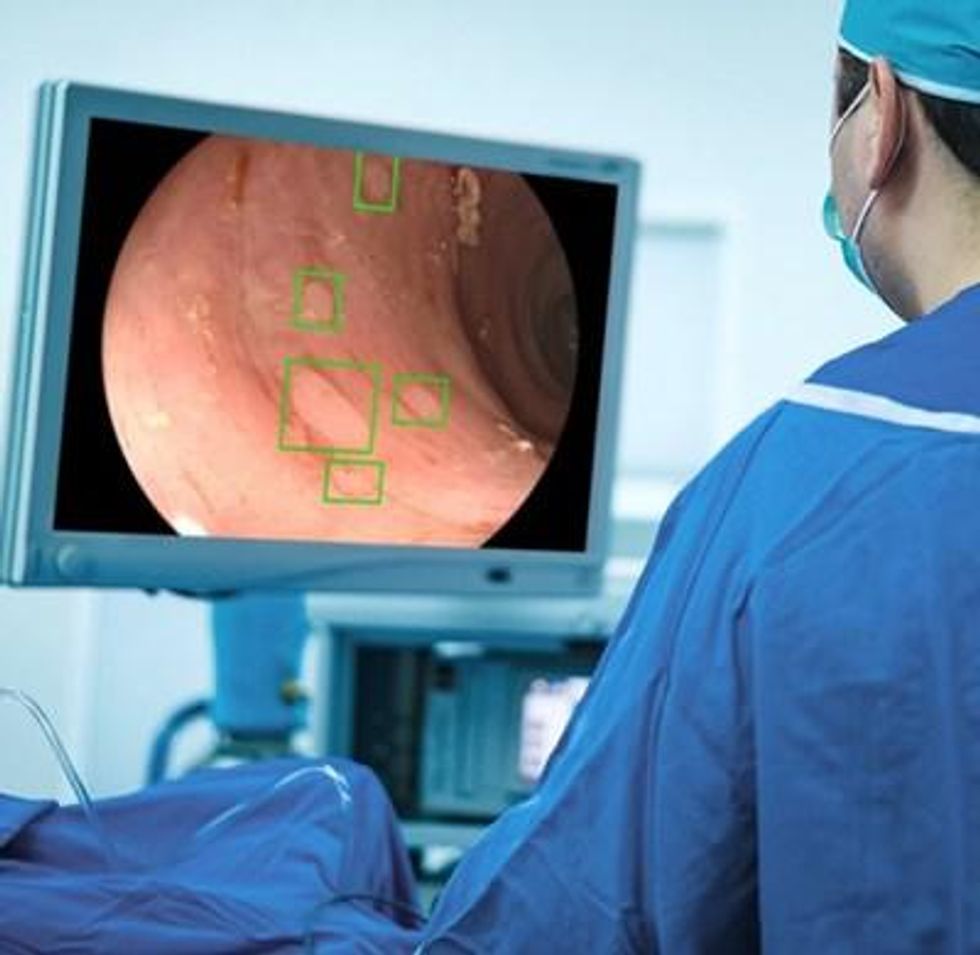
Medtronic Canada ULC, a subsidiary of Medtronic plc (NYSE: MDT) — the global leader in medical technology — announced it has received a Health Canada licence for the GI Genius™ intelligent endoscopy module. GI Genius is a computer-aided detection (CADe) system that uses artificial intelligence (AI) to highlight regions of the colon suspected to have visual characteristics consistent with different types of mucosal abnormalities.
Compatible with any colonoscope video, the module provides physicians with a powerful tool in the fight against colorectal cancer — the third most common form of cancer in Canada with 26,300 cases diagnosed in 2019. i
"More than a million screening colonoscopies are performed in Canada each year. The integration of leading-edge technologies such as AI into gastroenterology practices is very exciting," said Dr. Clarence Wong , section chief, gastroenterology, Alberta Health Services, Edmonton Zone. "It could be a key factor in the prevention of colorectal cancer."
The GI Genius module uses advanced AI to highlight the presence of possible precancerous lesions with a visual marker in real-time. The colonoscopy images are processed using advanced algorithms that can identify and mark polyp abnormalities, including those that could otherwise go undetected by the human eye.
Acting as a second observer, the GI Genius module helps increase the adenoma detection rate (ADR). Studies show that every 1% increase in ADR reduces the risk of colorectal cancer by 3%. ii,iii The flexibility of this platform means it can be seamlessly integrated into different clinical workflows, enabling clinical decision making, and increasing quality of care by reducing variability.
"Detection of colonic adenomatous polyps during colonoscopy is an important quality metric," said Dr. Wong. "The addition of AI to help improve adenoma detection rates can increase the quality of colonoscopies, potentially improving diagnosis and outcomes for colon cancer patients."
Use of the GI Genius module in studies has resulted in a 14% absolute increase in ADR compared to colonoscopy alone for both flat (42% increase) and polyploid (36% increase) lesions, demonstrating increasing accuracy to help reduce the rise of interval cancers occurring between colonoscopies. iv
"Medtronic is committed to helping to improve outcomes for patients with colon cancer," said Neil Fraser , president of Medtronic Canada. "GI Genius is currently the only colonoscope-agnostic AI platform designed to enhance polyp detection. Simply put, this technology will help save lives by improving colon cancer detection rates."
Medtronic is the exclusive worldwide distributor of the GI Genius module, which was developed and is manufactured by Cosmo Pharmaceuticals.
Learn more at Medtronic.ca/gigenius
About Medtronic Canada ULC
Bold thinking. Bolder actions. We are Medtronic. Proud to serve Canadian healthcare for over 50 years, Medtronic Canada ULC is headquartered in Brampton, Ontario , with regional offices in Montreal and Vancouver , and is a subsidiary of Medtronic plc. We are the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people. Our technologies and therapies address 70 health conditions and include cardiac devices, cranial and spinal robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for all. In everything we do, we are engineering the extraordinary.
For more information on Medtronic Canada, visit www.Medtronic.ca and follow @MedtronicCa on Twitter and LinkedIn .
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the U.S. Securities and Exchange Commission. Actual results may differ materially from anticipated results.
| __________ | |
| i | Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2019. Toronto, ON: Canadian Cancer Society; 2019. |
| ii | Wang P, Berzin TM, Glissen Brown JR , et al. Gut 2019; 68:1813-1819. |
| iii | Corley DA, Jenson CD, Marks AR JR, et al. NEJM 2014;370:1298-306. |
| iv | Repici, A., Badalamenti, M., Maselli, R., et al. Gastroenterology 2020; doi: https://doi.org/10.1053/j.gastro.2020.04.062 . |
SOURCE Medtronic Canada ULC