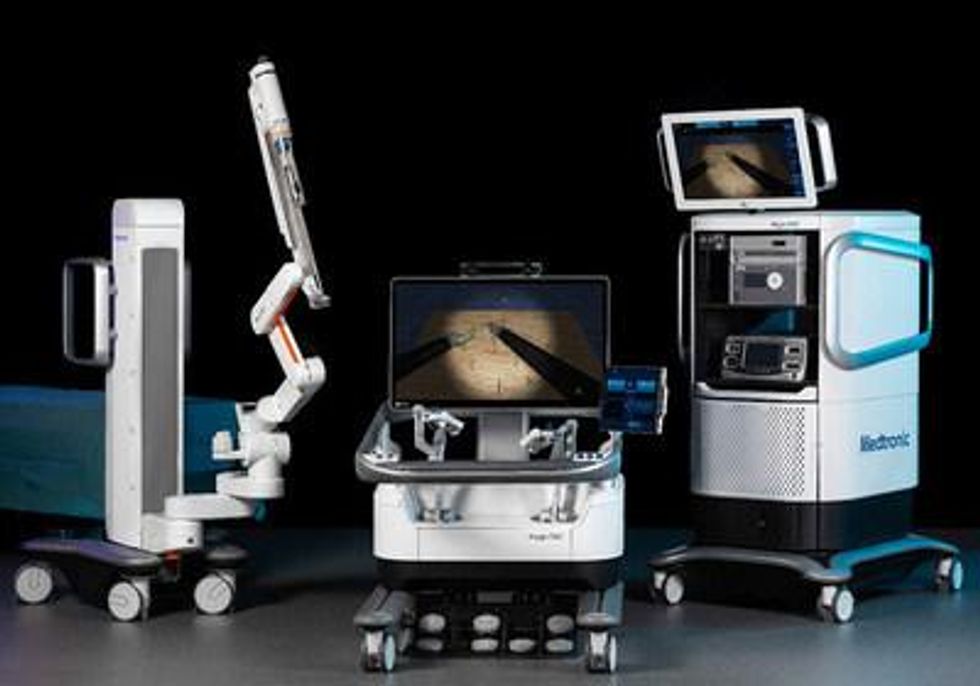
Medtronic Canada ULC, a subsidiary of Medtronic plc (NYSE: MDT), the world's leading medical technology company, announced it has received a Health Canada licence for the Hugo™ robotic-assisted surgery (RAS) system for use in use in urologic and gynecologic laparoscopic surgical procedures, which make up about half of all robotic procedures performed today. 1
"This licence ushers in a new opportunity for healthcare in Canada , bringing the benefits of robotic-assisted surgery to more patients by addressing the historic cost and utilization barriers that have stifled robotic surgery adoption for two decades," said Megan Rosengarten , president of the Surgical Robotics business, which is part of the Medical Surgical Portfolio at Medtronic. "We're beginning to see what the Hugo RAS system can do in the hands of clinicians in Latin America and Asia Pacific , and we're excited to see the possibilities it creates in Canada ."
Globally, about 3% of soft tissue surgeries are performed robotically, 1 despite offering patients the benefits of minimally invasive surgery — fewer complications, shorter hospital stays, and faster return to normal activities. 3–5,†
"Minimally invasive technology has a role to play in addressing the backlog of surgery in Canada . It can help optimize our precious healthcare human resources when patients spend less time in and out of the hospital, " said Neil Fraser , president of Medtronic Canada. "Today in Canada , only 1–2% percent of surgeries are performed with robotic assistance. 2 I'm proud that the launch of the Hugo system means we can help change that, and, more importantly, help to improve the patient and healthcare provider experience. Our first step will be to work with hospital partners to identify the best candidates for RAS based on patient outcomes and cost."
A modular, multi-quadrant platform indicated for urologic surgical procedures and gynecologic laparoscopic surgical procedures, the Hugo RAS system combines wristed instruments, 3D visualization, and a cloud-based surgical video capture option in Touch Surgery™ Enterprise with dedicated support teams specializing in robotics program optimization, service, and training.
Health Canada licensing comes on the heels of major milestones in the Hugo RAS system global launch, including the receipt of CE Mark approval in Europe and the first urologic and gynecologic cases in Latin America and India , which are included in the Hugo™ RAS system patient registry.
The Hugo RAS system is commercially available in certain geographies. Regulatory requirements of individual countries and regions will determine approval, clearance, or market availability. In the EU, the Hugo RAS system is CE marked. In Canada , the Hugo RAS system has a Health Canada licence. In the U.S., the Hugo RAS system is an investigational device not for sale. Touch Surgery Enterprise is not intended to direct surgery, or aid in diagnosis or treatment of a disease or condition.
About Medtronic Canada ULC
Bold thinking. Bolder actions. We are Medtronic. Proud to serve Canadian healthcare for over 50 years, Medtronic Canada ULC is headquartered in Brampton, Ontario , with regional offices in Montreal and Vancouver , and is a subsidiary of Medtronic plc. We are the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people. Our technologies and therapies address 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for all. In everything we do, we are engineering the extraordinary.
For more information on Medtronic Canada, visit www.Medtronic.ca
Follow us: @MedtronicCa on Twitter , and LinkedIn .
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the U.S. Securities and Exchange Commission. Actual results may differ materially from anticipated results.
| † | Compared to open surgery. |
| 1 | Based on internal estimates and Medtronic report, FY20 market model: procedural volume data. |
| 2 | Canadian Institute for Health Information. 2017-2018. Discharge Abstract Database 2017-2018 & National Ambulatory Care Reporting System 2017-2018. Purchased data set on file with Medtronic Canada. |
| 3 | Fitch K, Engel T, Bochner A. Cost differences between open and minimally invasive surgery. Managed Care. 2015 Sep;24(9):40–48. |
| 4 | Tiwari MM, Reynoso JF, High R, Tsang AW, Oleynikov D. Safety, efficacy, and cost effectiveness of common laparoscopic procedures. Surg Endosc. 2011;25(4):1127-1135. |
| 5 | Roumm AR, Pizzi L, Goldfarb NI, Cohn H. Minimally invasive: minimally reimbursed? An examination of six laparoscopic surgical procedures. Surg Innov. 2005;12(3):261–287. |
SOURCE Medtronic Canada ULC