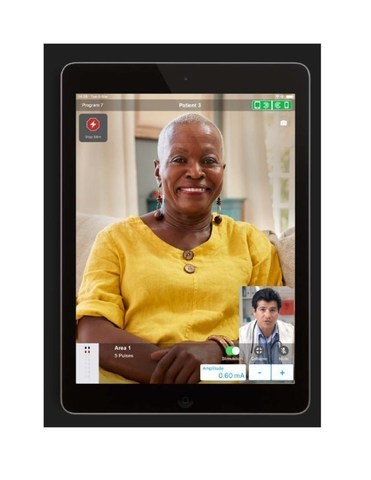
- The new system allows patients implanted with an Abbott neuromodulation device to communicate with their physician and remotely receive treatment in real time, regardless of location §
Abbott (NYSE: ABT) announces the Health Canada licencing† of NeuroSphere™ Virtual Clinic, a remote programming technology that is the first of its kind in Canada and is compatible with Abbott's suite of neuromodulation technologies. NeuroSphere Virtual Clinic has the potential to increase access to optimal treatment for patients living with chronic pain, Parkinson's disease, or essential tremors who might otherwise have difficulty receiving care from their healthcare provider due to location or being unable to travel to seek care.
Abbott's NeuroSphere Virtual Clinic gives patients the flexibility and comfort of receiving care anywhere § by connecting with their doctor via secure in-app video chat and an integrated remote programming feature. This feature enables clinicians to prescribe new treatment settings remotely to the patient's neurostimulation device using the clinician programmer app and a new, simple, and secure remote care connection.
In Canada , it is estimated that more than 6 million people live with chronic pain, 1 almost 100,000 people live with Parkinson's disease, 2 and almost 2 million people live with an essential tremor. 3 , 4 Many of these people who don't live close to a healthcare centre have difficulty accessing care due to the inability to see their doctor in-person. They are often challenged by the time associated with the trip, and/or the availability of a caregiver to help with their travel. In addition to the time implications, patients and caregivers may experience considerable travel-related costs and reduced employment wages. This is particularly true for those living in rural, northern, and remote parts of the country. 5
"Without alternatives to in-person programming, many of these patients may delay or forego care, particularly those who face a travel burden," said Alfonso Fasano , M.D., Ph.D., with Krembil Brain Institute at Toronto Western Hospital, part of the University Health Network. "Fortunately, innovative virtual healthcare options are changing the treatment landscape, ultimately extending care beyond clinic walls. Remote programming is an important new option that allows patients to communicate with their physicians virtually to ensure proper device settings and functionality. This brings the convenience of connected care to neurostimulation therapy, giving patients the ability to manage their therapy in a way that fits their personal needs."
NeuroSphere Virtual Clinic is compatible with the following Canadian-licensed Abbott neuromodulation devices: **
- Proclaim™ spinal cord stimulation (SCS), for the management of chronic, intractable pain of the trunk and/or limbs
- Proclaim dorsal root ganglion (DRG) therapy, for the management of moderate to severe chronic intractable pain of the lower limbs
- Infinity™ deep brain stimulation (DBS) therapy, for the management of Parkinson's disease and tremor
"For more than a decade, Abbott has been evaluating the treatment hurdles neuromodulation patients faced," said Pedro Malha, vice president, neuromodulation, Abbott. "During this time, we have diligently worked to find better ways of connecting patients to their doctors. The launch of NeuroSphere Virtual Clinic in Canada exemplifies Abbott's ability to put science and innovation to work, delivering solutions to help people live their best lives."
The ongoing evolution of Abbott neuromodulation technologies highlights the commitment to patient-centered research integrated with cutting-edge technologies to go beyond physical symptom relief and improve the lives of people with neurological disorders. Abbott's NeuroSphere Virtual Clinic was first launched in the United States in March 2021 .
*Neurostimulation systems for DBS are used in patients with levodopa-responsive Parkinson's disease or tremor. Please refer to the device Instructions for Use for details.
§ Anywhere with a cellular or Wi-Fi connection and sufficiently charged patient controller.
†The St. Jude Medical Clinician Programmer App and St. Jude Medical Patient Controller App used with the NeuroSphere Virtual Clinic are currently licensed.
**Certain configurations of the devices within may not have been licensed in accordance with Canadian law. Contact your local sales representative for the regulatory status of the device(s) in Canada . This product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use, inside the product carton (when available) or at medical.abbott/manuals for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events as applicable to Canada .
About Neuromodulation
Neuromodulation is an advanced personalized treatment option often recommended for patients who suffer from chronic pain and certain movement disorders, such as Parkinson's disease and essential tremors. Abbott neuromodulation systems use a recharge-free implanted generator, and a thin wire called a lead to send mild electrical pulses to areas of the brain that cause pain or tremors. These pulses interrupt the signals responsible for these symptoms. 6
About Abbott
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 113,000 colleagues serve people in more than 160 countries.
Connect with us at www.abbott.com , on LinkedIn at www.linkedin.com/company/abbott-/ , on Facebook at www.facebook.com/Abbott and on Twitter @AbbottNews.
1 Prevalence of chronic pain among individuals with neurological conditions. Statistics Canada . Updated March 21, 2018 . Accessed on May 25, 2022 . https://www150.statcan.gc.ca/n1/pub/82-003-x/2018003/article/54921-eng.htm
2 About Parkinson's Disease. Parkinson's Canada . Accessed on May 25, 2022 . https://www.parkinson.ca/about-parkinsons/
3 Agarwal, S. (2020, July 14 ). Essential Tremor. Access on May 25, 2022 . https://www.ncbi.nlm.nih.gov/books/NBK499986/
4 Canada's population estimates: Age and sex, July 1, 2021 . Statistics Canada . Updated September 29, 2022 . Accessed May 25, 2022 . https://www150.statcan.gc.ca/n1/daily-quotidien/210929/dq210929d-eng.htm
5 Publicly funded medical travel subsidy programs in Canada . Canadian Social Work Review. Volume 34, Number 1, 2017, p. 123–139. Accessed May 24, 2022 . https://www.erudit.org/en/journals/cswr/1900-v1-n1-cswr03182/1040998ar/
6 Yu, H., & Neimat, J. (2008). The treatment of movement disorders by deep brain stimulation. Neurotherapeutics , 5, 26-36 https://dx.doi.org/10.1016/j.nurt.2007.10.072 .
SOURCE Abbott

![]() View original content to download multimedia: https://www.newswire.ca/en/releases/archive/July2022/26/c0548.html
View original content to download multimedia: https://www.newswire.ca/en/releases/archive/July2022/26/c0548.html