
December 18, 2024
Founded by a team of seasoned entrepreneurs and healthcare experts, Zero Candida (TSXV:ZCT) is a fem-tech pioneer, combining advanced artificial intelligence with non-drug diagnostics and personalized treatment modalities in a single device. The company developed an innovative, first-of-its-kind solution to the diagnosis and treatment of Candidiasis, a fungal infection causing irritation, discharge and intense itchiness of the vagina and the vulva. The device offers precision therapy by eliminating fungal infections with over 99.99 percent effectiveness in just three hours, a revolutionary improvement over existing treatments.
Zero Candida is on track to complete clinical trials and file for FDA approval in 2024 with its innovative technology.
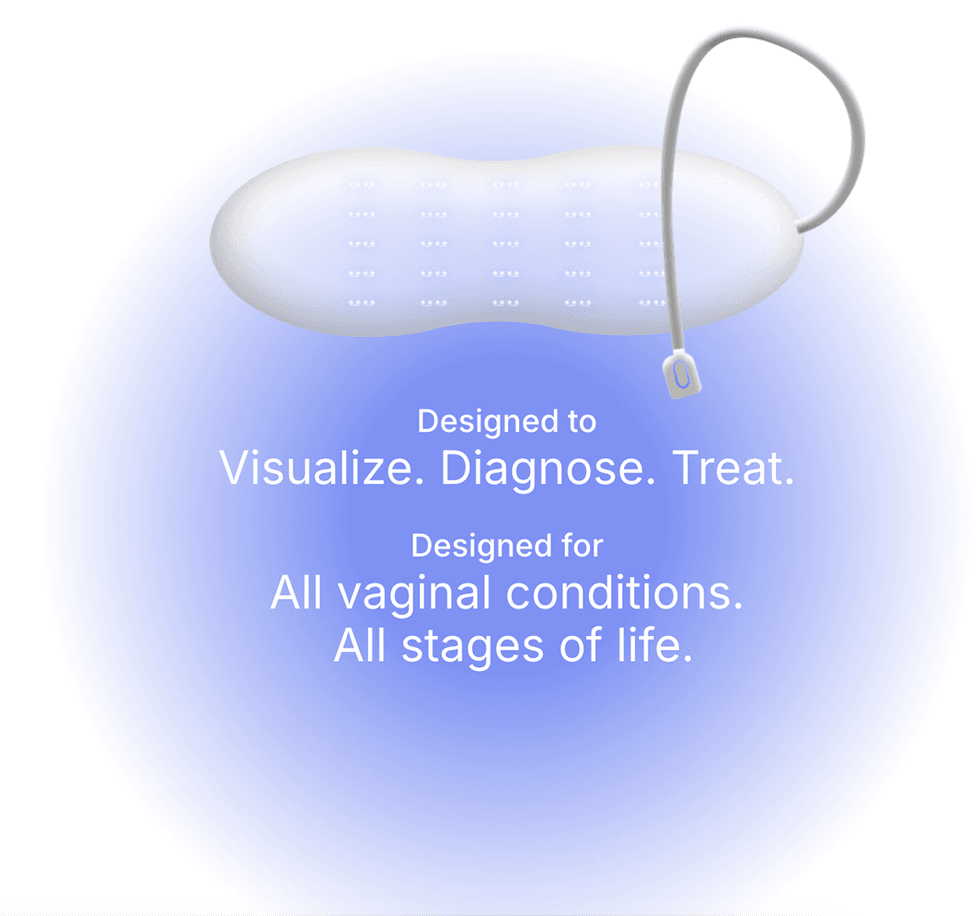
Zero Candida's SMART vaginal diagnostic device utilizes blue light therapy to treat this widespread condition without drugs. This non-drug therapy addresses key issues associated with conventional antifungal medications, including reduced risk of drug resistance, minimized side effects, and potential for faster symptom relief
Company Highlights
- Zero Candida Technologies is a fem-tech company focused on developing a SMART diagnostic and therapeutic device aimed at eliminating vaginal candidiasis (commonly known as yeast infection), a condition that affects three out four women globally.
- Candidiasis a fungal infection causing irritation, discharge and intense itchiness of the vagina and the vulva. In several cases, the current treatment for Candidiasis has been ineffective.
- Zero Candida has completed proof-of-concept studies, and demonstrated near-complete fungal eradication with over 99.99 percent effectiveness in just three hours.
- Founded by a team of experienced entrepreneurs and healthcare experts, the company is addressing the significant unmet needs of the women’s health market.
- The fem-tech segment of the med tech market is expected to grow at a CAGR of 18.2 percent and is estimated to reach nearly US$30 billion by 2032. North America dominated the global fem-tech market with a share of 52.91 percent in 2023.
- The company has already patented this technology in South Africa, while additional filings for patent application in the US and EU are underway.
This Zero Candida profile is part of a paid investor education campaign.*
Click here to connect with Zero Candida (TSXV:CZT) to receive an Investor Presentation
ZCT:CC
The Conversation (0)
16 December 2024
Zero Candida Technologies
Pioneering a technology-driven, innovative solution for the non-drug treatment of Candidiasis.
Pioneering a technology-driven, innovative solution for the non-drug treatment of Candidiasis. Keep Reading...
28 January
Seegnal Presents Real-World Evidence on Reducing Fall Risk in Geriatric Patients at Caltcm Summit
Seegnal Inc. (TSXV: SEGN) ("Seegnal" or the "Corporation"), a global leader in AI-enhanced prescription intelligence, today announced real-world clinical results demonstrating how medication governance may reduce fall-risk drivers in older adults -- a significant clinical and financial challenge... Keep Reading...
05 January
Pathways to Commercialising Biotech Innovations
In the medical technology industry, innovation is only the first step. While key to long-term success, innovation is only as good as a company’s commercialisation strategy. Once a technology has been developed and proven, the organisation must then embark on a process to commercialise it for... Keep Reading...
25 February 2025
HeraMED Signs Strategic Collaboration Agreement with Garmin Health
HeraMED Limited (ASX: HMD), a medical data and technology company leading the digital transformation of maternity care, is delighted to announce it has entered into a collaboration agreement with Garmin (NYSE: GRMN), a leading global provider of smartwatches and GPS-enabled products, aimed at... Keep Reading...
23 January 2025
Cyclopharm Signs US Agreement with HCA Healthcare for Technegas®
Cyclopharm Limited (ASX: CYC) is pleased to announce the signing of a major contract with Hospital Corporation of America Healthcare (HCA), one of the largest single healthcare providers in the United States. This agreement marks a significant milestone for the company which will allow the... Keep Reading...
23 January 2025
CONNEQT App Launches in USA as Pulse Deliveries Commence
Cardiex Limited (CDX:AU) has announced CONNEQT App Launches in USA as Pulse Deliveries CommenceDownload the PDF here. Keep Reading...
16 January 2025
Revolutionizing Women's Health: Antifungal Innovation Brings New Investment Opportunities
The intersection of women's health and antifungal innovation represents a pivotal moment in healthcare, offering both transformative medical advancements and compelling investment opportunities. The groundbreaking developments in antifungal treatments specifically targeting women's health issues... Keep Reading...
Latest News
Interactive Chart
Latest Press Releases
Related News
TOP STOCKS
American Battery4.030.24
Aion Therapeutic0.10-0.01
Cybin Corp2.140.00





