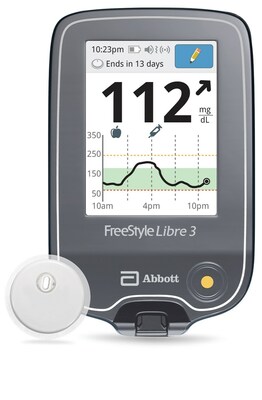
- The FreeStyle Libre 3 system is the latest generation in Abbott's FreeStyle Libre portfolio – the most prescribed and affordable integrated continuous glucose monitoring (iCGM) system in the United States 1, 2
- With a standalone reader, Abbott is working 3 to have the FreeStyle Libre 3 system available to Medicare beneficiaries who use insulin 4
Abbott (NYSE: ABT) today announced that the U.S. Food and Drug Administration (FDA) has cleared a reader for its FreeStyle Libre ® 3 integrated continuous glucose monitoring (iCGM) system, which features the world's smallest, thinnest and most discreet 5 glucose sensor. With the FDA's clearance of a standalone reader, Abbott is working to get the FreeStyle Libre 3 system added to Medicare's list of covered systems as soon as possible. 3
"Our customers all over the world consistently tell us how our FreeStyle Libre technology has made an enormous, positive impact on their health and quality of life – they spend less time worrying and more time living," said Jared Watkin , senior vice president for Abbott's diabetes care business. "The FreeStyle 3 reader provides more choice to people living with diabetes to have access to lifesaving technology that is smaller and easier to use and comes without the high-cost burdens of other systems."
The FreeStyle Libre 3 reader is a small handheld device that displays real-time glucose readings directly from a small sensor worn on the back of a person's upper arm, allowing them to manage their diabetes quickly and easily by viewing their glucose readings 6 on a large, bright and easy-to-see screen.
People who use the FreeStyle Libre 3 system will still have the option to use the current FreeStyle Libre 3 smartphone apps. 7,8
The reader uses a rechargeable lithium-ion battery, which is commonly found in many other electronic devices like mobile phones. The user manual for the FreeStyle Libre 3 reader provides details on how to safely store, charge and use the device, including always using the Abbott-provided USB cable and power adapter.
The FreeStyle Libre portfolio is the number one sensor-based glucose monitoring system in the world 9 , having changed the lives of 4.5 million people across more than 60 countries 10 by providing breakthrough technology that is accessible and affordable. 2
Important Safety Information
Failure to use the FreeStyle Libre 3 system as instructed in labeling may result in missing a severe low or high glucose event and/or making a treatment decision, resulting in injury. If glucose alarms and readings do not match symptoms or expectations, use a fingerstick value from a blood glucose meter for treatment decisions. Seek medical attention when appropriate or contact Abbott at 855-632-8658 for safety info.
About Abbott
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 115,000 colleagues serve people in more than 160 countries.
Connect with us at www.abbott.com , on LinkedIn at www.linkedin.com/company/abbott- , on Facebook at www.facebook.com/Abbott and on Twitter @AbbottNews .
- Data on file, Abbott Diabetes Care. Based on total active patients with Medicare CGM readers and the number of patients assigned to each manufacturer based on last filed prescription data in U.S. Retail Pharmacies and Durable Medical Equipment Suppliers.
- Based on a comparison of list prices of the FreeStyle Libre 3 iCGM system versus competitors' (i)CGM systems. The actual cost to patients may or may not be lower than other (i)CGM systems, depending on the amount covered by insurance, if any. Abbott provides this information as a courtesy; it is subject to change and interpretation. The customer is ultimately responsible for determining the appropriate codes, coverage, and payment policies for individual patients. Abbott does not guarantee third-party coverage or payment for our products or reimburse customers for claims that are denied by third-party payers.
- Abbott is in process of filing a standard code verification request with the Medicare Pricing Data Analysis and Coding (PDAC) contractor to assign the FreeStyle Libre 3 system to the appropriate CGM durable medical equipment (DME) code, as required for Medicare coverage. See Glucose Monitor – Policy Article A52464, available at https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleid=52464&ver=43& .
- Centers for Medicare & Medicaid Service Local Coverage Determination Coverage Guidance L33822 from the Medicare Administrative Contractors https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=33822 . Published and accessed on March 2 , 2023. For coverage of a CGM, patients must be diagnosed with diabetes; have sufficient training on using a CGM based on their healthcare provider's conclusion; be prescribed a CGM device in accordance with its FDA indications for use; use insulin or have a history of problematic hypoglycemia; have seen their healthcare provider in-person or through a Medicare-approved telehealth visit to evaluate their diabetes control within six months before the CGM was ordered; and every six months following their initial CGM prescription must go in-person or have a Medicare-approved telehealth visit with their healthcare provider to document their CGM adherence and diabetes treatment plan.
- Among patient-applied sensors.
- To start a new sensor, a user must hold their reader up to the sensor for an initial scan. After the initial scan and warm up period, data from the sensor sends readings directly to the reader every minute. Refer to the FreeStyle Libre 3 reader user's manual for further instructions.
- If users start a new sensor with the reader, they will not be able to use their smartphone app to check their glucose levels for the entire sensor wear period. If a user wishes to switch to their smartphone app to check their glucose levels, they must start a new sensor.
- The FreeStyle Libre 3 app is only compatible with certain mobile devices and operating systems. Please check our website for more information about device compatibility before using an app.
- Data based on the number of users worldwide for FreeStyle Libre family of personal CGMs compared to the number of users for other leading personal CGM brands and based on CGM sales dollars compared to other leading personal CGM brands.
- Data on file, Abbott Diabetes Care.
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/fda-clears-reader-for-abbotts-freestyle-libre-3-system-301797385.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/fda-clears-reader-for-abbotts-freestyle-libre-3-system-301797385.html
SOURCE Abbott