
November 05, 2023
Ovarian cancer diagnostics company, Cleo Diagnostics Limited (ASX:COV) (CLEO, or the Company) is pleased to announce the publication of an article on its triage test for ovarian cancer.
Highlights
- Results from the first clinical validation study of the CleoDX Triage Test, performed in a 334 patient cohort, have been published in the peer-reviewed journal “Cancers”
- The article, entitled ‘A novel predictive multi-marker test for the pre-surgical identification of ovarian cancer’ provides a detailed overview of the high performance of Cleo’s ovarian cancer diagnostics test
- The article concluded that Cleo’s ovarian cancer test:
- Was highly accurate with 95% sensitivity1 / 95% specificity2;
- Correctly discriminated malignant from benign samples; and
- Out-performed and was superior to current clinical methods.
- Peer-review provides important validation of Cleo’s technology and commercial strategy, targeting the surgical triage market where accurate and sensitive identification of malignant tumours is essential.
PEER REVIEWED PUBLICATION
Cleo’s first clinical validation study for its ovarian cancer triage test has been published in the peer- reviewed international journal ‘Cancers’. The results confirm the high accuracy of the CleoDX Triage Test, which was independent of menopausal status, and showed that it out-performed the two most widely used clinical scoring systems (the “Risk of Malignancy Index” and “Risk of Malignancy Algorithm”) for discriminating benign from malignant ovarian disease.
A copy of the publication is available here: https://www.mdpi.com/2072-6694/15/21/5267
Moreover, the CleoDX surgical triage test correctly identified 81% of early-stage cancer patients in the cohort.
Commenting on the publication, Cleo Chief Scientific Officer, Dr Andrew Stephens, said:
“These results confirm that our core technology is robust and accurate, and most importantly can identify cancers at an early stage. These results strongly support our planned further development of this core technology aimed at ovarian cancer screening in the longer term”.
CLEO Chief Executive, Richard Allman, added:
“This is an important step forward as we work towards our goal of an FDA approved triage test. I look forward to providing further updates to the market as we progress through our development program.”
The next step in development will confirm functionality of the commercially available kits in an independent clinical trial, the results of which will be submitted to the FDA for regulatory approval. Cleo anticipates commencement of this trial before the end of CY 2023.
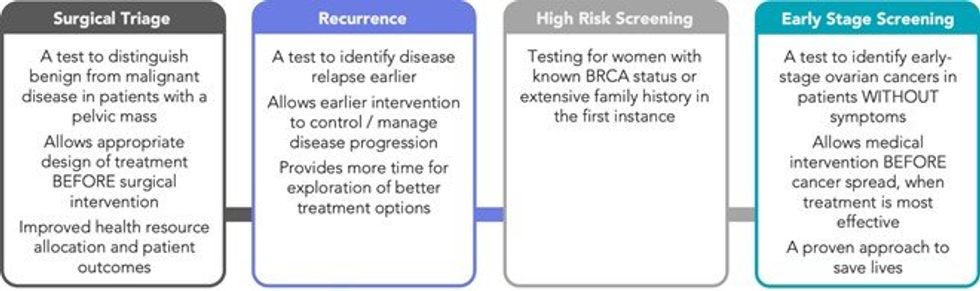
OPPORTUNITY FOR CLEO AND OVARIAN CANCER DIAGNOSTICS MARKET
At present there is no clinically routine pre-surgical method for reliable evaluation and differentiation of benign vs malignant ovarian cancer tumours. Radical surgery is the cornerstone of cancer management, with complete hysterectomy being the norm. Removal of the ovaries, however, predisposes women to multiple co-morbidities including increased risk of cardiovascular disease, dementia and certain cancers amongst others. There is a clear need to differentiate benign vs malignant cases pre-surgically to enhance patient outcomes.
Cleo has defined a staged execution strategy to deliver it’s simple blood test which is focused on three key markets across pre-surgical triage testing, high-risk/recurrence detection, and broader screening programs. Achieving a positive outcome here from a peer-reviewed publication, has a material impact on the Company’s pathway with respect to the initial triage market. The Company will now use the publication of its test performance to further define the scope of the triage market.
Click here for the full ASX Release
This article includes content from CLEO Diagnostics, licensed for the purpose of publishing on Investing News Australia. This article does not constitute financial product advice. It is your responsibility to perform proper due diligence before acting upon any information provided here. Please refer to our full disclaimer here.
COV:AU
The Conversation (0)
21 May 2024
Cleo Diagnostics
Revolutionising Ovarian Cancer Diagnosis Through Accurate and Early Detection
Revolutionising Ovarian Cancer Diagnosis Through Accurate and Early Detection Keep Reading...
17 March 2025
CLEO Delivers Commercial Development Milestone
Cleo Diagnostics (COV:AU) has announced CLEO Delivers Commercial Development MilestoneDownload the PDF here. Keep Reading...
27 February 2025
Appendix 4D and Half Yearly Accounts
Cleo Diagnostics (COV:AU) has announced Appendix 4D and Half Yearly AccountsDownload the PDF here. Keep Reading...
31 January 2025
Quarterly activities and cashflow report
Cleo Diagnostics (COV:AU) has announced Quarterly activities and cashflow reportDownload the PDF here. Keep Reading...
09 December 2024
CLEO Further Expands Ovarian Cancer Trial with Siles Health
Cleo Diagnostics (COV:AU) has announced CLEO Further Expands Ovarian Cancer Trial with Siles HealthDownload the PDF here. Keep Reading...
26 November 2024
The Royal Women's Hospital Joins CLEO Ovarian Cancer Trial
Cleo Diagnostics (COV:AU) has announced The Royal Women's Hospital Joins CLEO Ovarian Cancer TrialDownload the PDF here. Keep Reading...
28 January
Seegnal Presents Real-World Evidence on Reducing Fall Risk in Geriatric Patients at Caltcm Summit
Seegnal Inc. (TSXV: SEGN) ("Seegnal" or the "Corporation"), a global leader in AI-enhanced prescription intelligence, today announced real-world clinical results demonstrating how medication governance may reduce fall-risk drivers in older adults -- a significant clinical and financial challenge... Keep Reading...
05 January
Pathways to Commercialising Biotech Innovations
In the medical technology industry, innovation is only the first step. While key to long-term success, innovation is only as good as a company’s commercialisation strategy. Once a technology has been developed and proven, the organisation must then embark on a process to commercialise it for... Keep Reading...
25 February 2025
HeraMED Signs Strategic Collaboration Agreement with Garmin Health
HeraMED Limited (ASX: HMD), a medical data and technology company leading the digital transformation of maternity care, is delighted to announce it has entered into a collaboration agreement with Garmin (NYSE: GRMN), a leading global provider of smartwatches and GPS-enabled products, aimed at... Keep Reading...
23 January 2025
Cyclopharm Signs US Agreement with HCA Healthcare for Technegas®
Cyclopharm Limited (ASX: CYC) is pleased to announce the signing of a major contract with Hospital Corporation of America Healthcare (HCA), one of the largest single healthcare providers in the United States. This agreement marks a significant milestone for the company which will allow the... Keep Reading...
23 January 2025
CONNEQT App Launches in USA as Pulse Deliveries Commence
Cardiex Limited (CDX:AU) has announced CONNEQT App Launches in USA as Pulse Deliveries CommenceDownload the PDF here. Keep Reading...
16 January 2025
Revolutionizing Women's Health: Antifungal Innovation Brings New Investment Opportunities
The intersection of women's health and antifungal innovation represents a pivotal moment in healthcare, offering both transformative medical advancements and compelling investment opportunities. The groundbreaking developments in antifungal treatments specifically targeting women's health issues... Keep Reading...
Latest News
Interactive Chart
Latest Press Releases
Obonga Project: Wishbone VMS Update
27 February
Related News
TOP STOCKS
American Battery4.030.24
Aion Therapeutic0.10-0.01
Cybin Corp2.140.00









