September 02, 2024
TruScreen Group Limited (NZX/ASX:TRU) is pleased to announce the preliminary publication, on 25 July 2024, of a study titled “Beyond Tradition: Investigating TruScreen’s Performance Versus Pap Smear in Cervical Cancer Detection” on Research Square1 Link. The preliminary publication is subject to peer review.
Highlights
- 507 women tested from 2021-2022, results published in July 2024
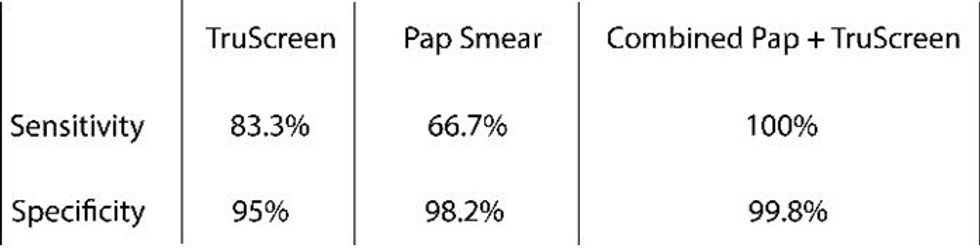
- High Sensitivity and Specificity: TruScreen demonstrated a sensitivity of 83.3% and a specificity of 95% for detecting cervical lesions (neoplasms), compared to 66.7% and 98.2% for Pap smear, respectively1.
- Real-Time Results: The TruScreen device provides immediate screening results, eliminating the need for laboratory equipment and pathology staff.
- Practical and Reliable: The study confirmed that TruScreen is a practical and reliable screening tool, suitable for use in various healthcare settings.
The authors concluded that TruScreen “represents a reliable, practical screening tool for cervical neoplasms” and that their results “provide an evidence-based approach for policymakers when selecting the optimal cervical cancer screening strategy in countries without an established national screening program.”
This study reinforces TruScreen’s commitment to providing innovative and accessible healthcare solutions. The positive results validate TruScreen’s technology and opens new opportunities in the global healthcare market.
For more information, please visit our website at www.truscreen.com.
Click here for the full ASX Release
This article includes content from Truscreen, licensed for the purpose of publishing on Investing News Australia. This article does not constitute financial product advice. It is your responsibility to perform proper due diligence before acting upon any information provided here. Please refer to our full disclaimer here.
The Conversation (0)
28 January
Seegnal Presents Real-World Evidence on Reducing Fall Risk in Geriatric Patients at Caltcm Summit
Seegnal Inc. (TSXV: SEGN) ("Seegnal" or the "Corporation"), a global leader in AI-enhanced prescription intelligence, today announced real-world clinical results demonstrating how medication governance may reduce fall-risk drivers in older adults -- a significant clinical and financial challenge... Keep Reading...
05 January
Pathways to Commercialising Biotech Innovations
In the medical technology industry, innovation is only the first step. While key to long-term success, innovation is only as good as a company’s commercialisation strategy. Once a technology has been developed and proven, the organisation must then embark on a process to commercialise it for... Keep Reading...
25 February 2025
HeraMED Signs Strategic Collaboration Agreement with Garmin Health
HeraMED Limited (ASX: HMD), a medical data and technology company leading the digital transformation of maternity care, is delighted to announce it has entered into a collaboration agreement with Garmin (NYSE: GRMN), a leading global provider of smartwatches and GPS-enabled products, aimed at... Keep Reading...
23 January 2025
Cyclopharm Signs US Agreement with HCA Healthcare for Technegas®
Cyclopharm Limited (ASX: CYC) is pleased to announce the signing of a major contract with Hospital Corporation of America Healthcare (HCA), one of the largest single healthcare providers in the United States. This agreement marks a significant milestone for the company which will allow the... Keep Reading...
23 January 2025
CONNEQT App Launches in USA as Pulse Deliveries Commence
Cardiex Limited (CDX:AU) has announced CONNEQT App Launches in USA as Pulse Deliveries CommenceDownload the PDF here. Keep Reading...
16 January 2025
Revolutionizing Women's Health: Antifungal Innovation Brings New Investment Opportunities
The intersection of women's health and antifungal innovation represents a pivotal moment in healthcare, offering both transformative medical advancements and compelling investment opportunities. The groundbreaking developments in antifungal treatments specifically targeting women's health issues... Keep Reading...
Latest News
Interactive Chart
Latest Press Releases
Related News
TOP STOCKS
American Battery4.030.24
Aion Therapeutic0.10-0.01
Cybin Corp2.140.00