Seelos Therapeutics, Inc. (Nasdaq: SEEL), a clinical-stage biopharmaceutical company focused on the development of therapies for central nervous system disorders and rare diseases, today presented a poster on Part 1, the open-label portion, of the study of SLS-002 (Intranasal Racemic Ketamine) at the IASRAFSP International Summit on Suicide Research Virtual Conference.
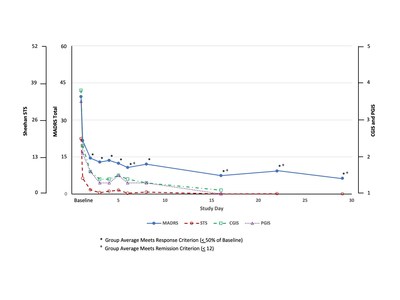
The poster titled A Phase 2 Open Label Study of Efficacy, Safety, and Tolerability of SLS-002 (Intranasal Racemic Ketamine) in Adults with Major Depressive Disorder at Imminent Risk of Suicide , demonstrated rapid, robust and sustained reductions on the Montgomery -Åsberg Depression Rating Scale (MADRS), the Clinical Global Impression of Severity for Suicidal Ideation and Behavior (CGIS-SI/B), the Patient Global Impression of severity for Suicidal Ideation and Behavior (PGIS-SI/B), and the Sheehan-Suicidality Tracking Scale (S-STS).
"SLS-002 demonstrated a rapid onset of action, impressive efficacy and a sustained improvement from the first dose through the end of the study, including the group mean meeting the MADRS remission criteria initially on Day 6 after only 2 doses," said Raj Mehra, Ph.D., Chairman and CEO of Seelos. "Meeting these early criteria for response and remission is highly encouraging considering that these patients were not only severely depressed but also acutely suicidal."
This open-label portion of the study enrolled 17 subjects diagnosed with major depressive disorder (MDD) requiring psychiatric hospitalization due to significant risk of suicide with a baseline score of > 28 points on the MADRS, a score of 5 or 6 on MADRS Item-10, a score of > 15 points on the S-STS total score and a history of previous suicide attempt(s), as confirmed on the Columbia Suicide Severity Rating Scale (C-SSRS) with a history of at least one actual attempt, or if the attempt was interrupted or aborted, is judged to have been serious in intent.
The conference is organized by the International Academy of Suicide Research (IASR) in partnership with the American Foundation for Suicide Prevention (AFSP) .
For additional information: https://suicideresearchsummit.org/
If you or a loved one are having thoughts of suicide, please seek immediate medical help, go to your nearest emergency room, or call the National Suicide Prevention Lifeline at 1-800-273-8255.
About SLS-002
SLS-002 is intranasal racemic ketamine with two investigational new drug applications ("INDs"). The lead program is focused on the treatment of ASIB in MDD. SLS-002 was originally derived from a Javelin Pharmaceuticals, Inc./Hospira, Inc. program with 16 clinical studies involving approximately 500 subjects. SLS-002 looks to address an unmet need for an efficacious drug to treat suicidality in the United States . Traditionally, anti-depressants have been used in this setting but many of the existing treatments are known to contribute to an increased risk of suicidal thoughts in some circumstances, and if and when they are effective, it often takes weeks for the full therapeutic effect to be manifested. We believe there is a large opportunity in the United States and European markets for products in this space. Based on information gathered from the databases of the Agency for Healthcare Research and Quality, there were approximately 1,000,000 visits to emergency rooms for suicide attempts in 2013 in the United States alone. Experimental studies suggest ketamine has the potential to be a rapid, effective treatment for refractory depression and suicidality.
About Seelos Therapeutics
Seelos Therapeutics, Inc. is a clinical-stage biopharmaceutical company focused on the development and advancement of novel therapeutics to address unmet medical needs for the benefit of patients with central nervous system (CNS) disorders and other rare diseases. The Company's robust portfolio includes several late-stage clinical assets targeting indications including Acute Suicidal Ideation and Behavior (ASIB) in Major Depressive Disorder (MDD) or Post-Traumatic Stress Disorder (PTSD), Amyotrophic lateral sclerosis (ALS), Sanfilippo syndrome, Parkinson's Disease, other psychiatric and movement disorders plus orphan diseases.
For more information, please visit our website: https://seelostherapeutics.com , the content of which is not incorporated herein by reference.
Forward Looking Statements
Statements made in this press release, which are not historical in nature, constitute forward-looking statements for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. These statements include, among others, those regarding Seelos' Phase 2 open label study of efficacy, safety, and tolerability of SLS-002 (Intranasal Racemic Ketamine) in adults with major depressive disorder at imminent risk of suicide (the "Study"), statements regarding SLS-002's prospects and statements regarding the Company's potential market opportunity. These statements are based on Seelos' current expectations and beliefs and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Risks associated with Seelos' business and plans described herein include, but are not limited to, the risk of not successfully executing its preclinical and clinical studies, or continuing the Study, and not gaining marketing approvals for its product candidates, the risk that prior clinical results may not be replicated in future studies and trials (including the risk that the clinical results from the Study are not replicated), the risks that clinical study results may not meet any or all endpoints of a clinical study and that any data generated from such studies may not support a regulatory submission or approval, the risks associated with the implementation of a new business strategy, the risks related to raising capital to fund its development plans and ongoing operations, risks related to Seelos' current stock price, risks related to the global impact of COVID-19, as well as other factors expressed in Seelos' periodic filings with the U.S. Securities and Exchange Commission, including its most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Contact Information:
Anthony Marciano
Chief Communications Officer
Seelos Therapeutics, Inc. (Nasdaq: SEEL)
300 Park Avenue
New York, NY 10022
(646) 293-2136
anthony.marciano@seelostx.com
https://seelostherapeutics.com/
https://twitter.com/seelostx
https://www.linkedin.com/company/seelos
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/seelos-therapeutics-presents-a-poster-on-sls-002-intranasal-racemic-ketamine-at-the-2021-iasrafsp-international-summit-on-suicide-research-301407611.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/seelos-therapeutics-presents-a-poster-on-sls-002-intranasal-racemic-ketamine-at-the-2021-iasrafsp-international-summit-on-suicide-research-301407611.html
SOURCE Seelos Therapeutics, Inc.