NurExone Biologic Inc. (TSXV: NRX,OTC:NRXBF) (OTCQB: NRXBF) (FSE: J90) (" NurExone " or the " Company ") a biopharmaceutical company developing exosome-based regenerative therapies, announced new preclinical results showing that its lead candidate ExoPTEN produces a reproducible, dose-dependent therapeutic effect in an eye model of glaucoma.
The study was conducted in collaboration with Prof. Ygal Rotenstreich team at the Goldschleger Eye Institute at Sheba Medical Center, one of the world's leading hospitals i . It demonstrated that ExoPTEN's biological activity increases with higher dosing levels in animals with optic nerve injury, resulting in consistent and measurable recovery of visual function. The findings show that ExoPTEN's regenerative effect is reproducible, quantifiable, and scales with dose.
"Reproducibility is the key challenge in science, and now we have a first validation of the results," said Prof. Michael Belkin, Professor Emeritus of Ophthalmology at Tel Aviv University and Scientific Advisor to NurExone. "These results confirm that ExoPTEN has the potential impact of a true therapeutic, producing reproducible repair of damaged optic nerves in small animals, advancing our ability to address vision loss in patients with optic nerve damage, such as glaucoma and related conditions."
Dr. Tali Kizhner, Director of Research and Development at NurExone, added: "We are seeing a clear, dose-dependent effect of ExoPTEN in the eye, with stronger functional recovery at higher doses. It highlights the one-two punch of our platform: exosomes that reach and protect neural tissue, and the siRNA cargo that switches on the regenerative response. This dose-response data marks an essential step toward future clinical trials and brings us closer to translating this therapy to patients."
This dose-response study, the third independent investigation of ExoPTEN's activity in optic nerve injury, complements the previously announced results from June 2024 and December 2024 , which showed structural preservation and survival of retinal ganglion cells.
The optic nerve crush ("ONC") model used in these experiments mimics the nerve damage that occurs in glaucoma, one of the leading causes of irreversible blindness. The researchers led by Prof. Rotenstreich, tested low and high doses of ExoPTEN delivered by extrachoroidal injection directly to the eye.
Functional measurements of retinal activity using scotopic threshold response electroretinography (STR-ERG) showed that both ExoPTEN doses improved visual signal strength in animals with optic nerve injury, with the high-dose group achieving response amplitudes comparable to those of uninjured eyes. This result demonstrates substantial functional recovery and provides clear evidence of a dose-dependent therapeutic effect that aligns with ExoPTEN's proposed biological mechanism.
"This collaboration opens a new treatment avenue for blinding glaucoma and other ophthalmological indications," commented Prof. Rotenstreich
Figure 1. Dose-dependent restoration of retinal response following ExoPTEN treatment in the ONC model.
The figure depicts scotopic threshold response (STR) amplitudes measured by electroretinography (ERG) in rats subjected to optic nerve crush (ONC) and treated with exosome-based formulations. The y-axis shows STR amplitude (µV), representing retinal ganglion cell function, while the x-axis displays experimental groups. Eyes with ONC were treated with low-dose or high-dose ExoPTEN (exosomes loaded with PTEN siRNA). Additional groups included eyes treated with naïve exosomes and uninjured eyes to establish baseline retinal response. In this model, visual responses are considered detectable when retinal signal amplitudes exceed about 5 µV ; signals below that level indicate no measurable retinal activity.
Each bar shows the mean STR amplitude ± standard error of the mean (SEM). Both ExoPTEN dose groups exhibited recovery of retinal electrical response relative to the expected ONC-induced decline, with the high-dose group achieving STR amplitudes comparable to those of uninjured eyes.
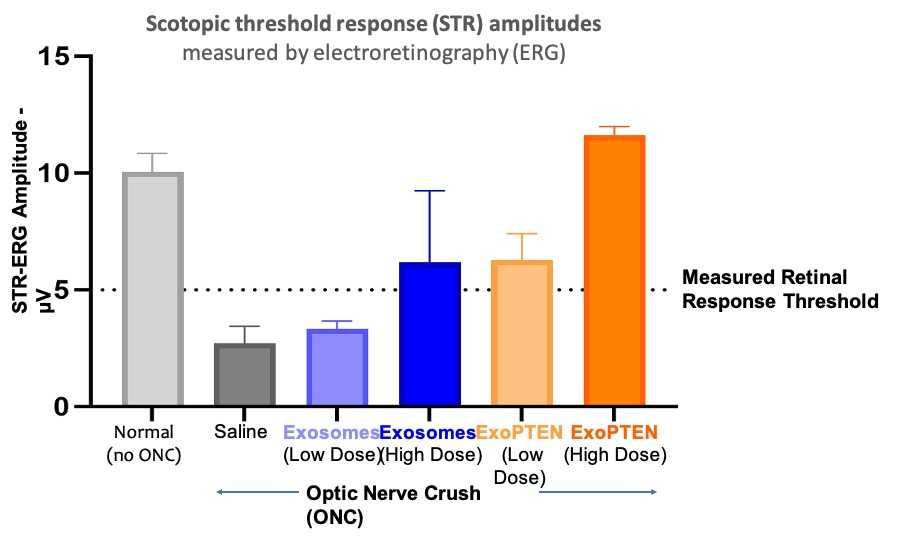
Figure 1 shows the amplitude of Electroretinogram (ERG) measurements of dark-adapted (scotopic) threshold retinal response (STR, in microvolts, µV) at 0.00062 cd/m^2. In each rat, one eye was left intact as a healthy control ("no ONC", light grey). The second eye had ONC and was treated according to group – treatment with PBS (vehicle, Saline, dark grey), with naïve exosomes (Exosomes, blue) or with ExoPTEN (ExoPTEN, orange). Naïve exosomes and ExoPTEN were given in low (4E+8 particles, light blue and orange) or high dose (4E+9 particles, dark blue or orange). Each treatment was given twice - right after the ONC surgery and 1 week after it.
A true response to light was considered any amplitude above 5µV. As can be seen, normal (no ONC) eyes (n=13) showed a clear response to the light in this low light intensity, while Saline-treated eyes show no response (n=6). In Exosome treated eyes, no eyes (n=2) responded to the light in low dose treatment, and only 1 of 2 high dose receiving eyes responded. In ExoPTEN-treated eyes, all eyes responded to the light, with a clear dose response shown by the higher, normal values, response of the high dose receiving eyes (n=2) compared to the lower dose receiving eyes (n=2).
Warrant Acceleration s
In addition, the Company is pleased to announce that further to its press releases dated August 28, 2023, September 6, 2023 and January 5, 2024, the Company is accelerating the expiration date (i) 2,515,456 class B Common Share (as defined herein) purchase warrants issued and outstanding from the private placement which closed in tranches on August 25, 2023 and September 6, 2023 (the " September 2023 Warrants ") and (ii) 5,653,073 Common Share purchase warrants issued and outstanding from the private placement which closed on January 4, 2024 (the " January 2024 Warrants ").
Each September 2023 Warrant is exercisable at a price of $0.48 per September 2023 Warrant and each January 2024 Warrant is exercisable at a price of $0.35 per January 2024 Warrant. If all of the September 2023 Warrants and January 2024 Warrants are exercised, the Company will receive total gross proceeds of approximately C$3.2 million . All funds received from exercises will be used for general corporate and working capital purposes.
The acceleration triggers were met after the daily volume weighted average trading price of the Company's common shares (" Common Shares ") on the TSX Venture Exchange (" TSXV ") equaled or exceeded for a period of 20 consecutive trading days, in the case of the September 2023 Warrants, $0.83, and in the case of the January 2024 Warrants, $0.80 (the " Acceleration Event ").
Pursuant to the terms of the warrants and following the occurrence of the Acceleration Event, the Company has provided notice to the warrant holders (the " Acceleration Notice "), thereby accelerating the expiry of the warrants. The warrants will now expire at 5:00 p.m. (Calgary time) on November 7 , 2025 , which is 30 days from the date of the Acceleration Notice. If the warrants are not exercised by such time, they will expire and be of no further force or effect.
Approximately 89 % of the Warrants are held by U.S.-based investors . The underlying shares issued upon exercise of these Warrants will remain restricted under applicable U.S. securities laws.
POSITIVE Communication s Agreement Extended
Further to the Company's press release dated April 10, 2025, the Company has, subject to TSXV approval, amended its agreement with POSITIVE Communications (" POSITIVE ") to: (i) extend the term to July 10, 2026, through the additional of a nine-month extension, and (ii) to add a monthly expense reimbursement of up to a maximum amount of NIS 15,000 plus VAT in addition to the monthly fee of NIS 15,000 plus VAT.
Either party has the right to terminate the agreement upon providing 30-days' notice. POSITIVE does not currently have a direct or indirect interest in the securities of the Company. While POSITIVE has no intention of acquiring any additional securities of the Company at this time, it may do so in the future in compliance with applicable securities laws and TSXV policies.
About NurExone
Nurexone Biologic Inc. is a TSXV, OTCQB, and Frankfurt-listed biotech company focused on developing regenerative exosome-based therapies for central nervous system injuries. Its lead product, ExoPTEN, has demonstrated strong preclinical data supporting clinical potential in treating acute spinal cord and optic nerve injury, both multi-billion-dollar markets. Regulatory milestones, including obtaining the Orphan Drug Designation, facilitates the roadmap towards clinical trials in the U.S. and Europe. Commercially, the Company is expected to offer solutions to companies interested in quality exosomes and minimally invasive targeted delivery systems for other indications. NurExone has established Exo-Top Inc., a U.S. subsidiary, to anchor its North American activity and growth strategy.
For additional information and a brief interview, please watch Who is NurExone? , visit www.nurexone.com or follow NurExone on LinkedIn , Twitter , Facebook , or YouTube .
For more information, please contact:
Dr. Lior Shaltiel
Chief Executive Officer and Director
Phone: +972-52-4803034
Email: info@nurexone.com
Dr. Eva Reuter
Investor Relations – Germany
Phone: +49-69-1532-5857
Email: e.reuter@dr-reuter.eu
Allele Capital Partners
Investor Relations – U.S.
Phone: +1 978-857-5075
Email: aeriksen@allelecapital.com
FORWARD-LOOKING STATEMENTS
This press release contains certain "forward-looking statements" that reflect the Company's current expectations and projections about its future results. Wherever possible, words such as "may", "will", "should", "could", "expect", "plan", "intend", "anticipate", "believe", "estimate", "predict" or "potential" or the negative or other variations of these words, or similar words or phrases, have been used to identify these forward-looking statements. Forward-looking statements in this press release include, but are not limited to, statements relating to: the a nticipated results, effects and benefits of ExoPTEN and other product candidates and their progression towards clinical trials and patients ; warrantholders exercising their warrants and the anticipated proceeds and use of proceeds ; receipt of all regulatory approvals , including the TSXV ; the extension and amendment of the POSITIVE agreement; POSITIVE acquiring securities in the Company in the future; and the NurExone platform technology offering novel solutions to drug companies interested in minimally invasive targeted drug delivery for other indications, including recovery of optic nerve function and overall visual health.
These statements reflect management's current beliefs and are based on information currently available to management as at the date hereof. In developing the forward-looking statements in this press release, we have applied several material assumptions, including: t he Company's ability to carry out preclinical and clinical studies and realize the anticipated results, effects, and benefits of ExoPTEN and other product candidates as they progress toward clinical trials and patients; the expectation that warrantholders will exercise their warrants and that the anticipated proceeds will be received and used as intended; the Company's ability to obtain and maintain all necessary regulatory approvals, including those from the TSXV; the successful extension and amendment of the POSITIVE agreement; the possibility that POSITIVE may acquire securities in the Company in the future in compliance with applicable laws and policies; and the ability of the NurExone platform technology to offer novel solutions to drug companies interested in minimally invasive targeted drug delivery for other indications, including recovery of optic nerve function and overall visual health.
Forward-looking statements involve significant risk, uncertainties and assumptions. Many factors could cause actual results, performance or achievements to differ materially from the results discussed or implied in the forward-looking statements. These risks and uncertainties include, but are not limited to risks related to: the Company's early stage of development and lack of revenues to date; the inherent uncertainty of preclinical drug development, including the risk that product candidates may not advance to clinical trials or receive regulatory approval; the possibility that results from preclinical studies and early-stage trials may not predict later outcomes; the uncertain timing, cost, and outcome of preclinical and clinical development activities; risks related to the clinical trial process, including potential delays or failure to achieve effective trial design or positive results; the inability to obtain or maintain required regulatory approvals, including from the TSXV; risks related to the anticipated proceeds and use of proceeds from warrant exercises; limited market acceptance of the Company's products, even if approved; the potential emergence of competing therapies that are safer, more effective, or more affordable; rapid technological change that may impact the relevance of the Company's technologies; the Company's dependence on key personnel and strategic partners; the inability to obtain adequate financing; risks related to the Company's ability to protect its intellectual property; the possibility that the Company's technologies, including its exosome-based platforms, may not achieve their intended therapeutic impact; the inability to produce or scale exosome-based products for clinical use; limited adoption in regenerative medicine or cell therapy applications; lack of growing clinical demand in targeted indications such as spinal cord injury, optic nerve repair, or other therapeutic areas; failure to meet planned development milestones or achieve commercial breakthroughs; the Company will not initiate additional studies to explore alternative dosing regimens ; the Company will not advance the optimization of ExoPTEN's manufacturing processes and analytical methods ; the Company will not prepare regulatory submissions; the Company will not launch first-in-human clinical trials ; the possibility that the NurExone platform technology may not offer novel solutions to drug companies interested in minimally invasive targeted drug delivery for other indications, including recovery of optic nerve function and overall visual health; and the risks discussed under the heading "Risk Factors" on pages 44 to 51 of the Company's Annual Information Form dated August 27, 2024, a copy of which is available under the Company's SEDAR+ profile at www.sedarplus.ca . These factors should be considered carefully, and readers should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in this press release are based upon what management believes to be reasonable assumptions, the Company cannot assure readers that actual results will be consistent with these forward-looking statements. These forward-looking statements are made as of the date of this press release, and the Company assumes no obligation to update or revise them to reflect new events or circumstances, except as required by law.
Neither TSXV nor its Regulation Services Provider (as that term is defined in the policies of the TSXV) accepts responsibility for the adequacy or accuracy of this release.
___________________________
i https://www.shebaonline.org/sheba-ranks-8-on-newsweeks-best-hospitals-list/
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/c7a05e34-0e2a-48cf-8ad6-9e6791fa025d