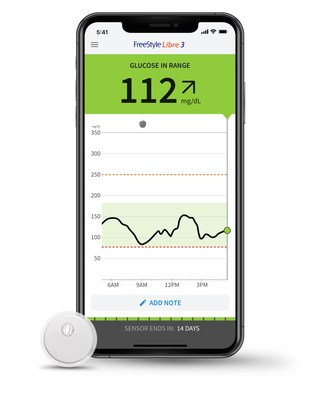
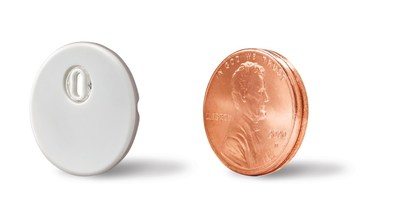- Abbott (NYSE: ABT), the worldwide leader in continuous glucose monitoring (CGM) technology, 8 announced today it has secured CE Mark (Conformité Européenne) for its next-generation FreeStyle Libre 3 system, which is now approved for use by people living with diabetes in Europe . The FreeStyle Libre 3 technology provides continuous, real-time glucose readings automatically delivered to smartphones 2 every minute, offering unsurpassed 14-day accuracy 1 in the smallest and thinnest 3 sensor design at the same affordable price 7 as previous versions of the device.
"Abbott won't stop innovating when there's room to raise the bar. We've done that again with FreeStyle Libre 3, the smallest sensor that delivers life-changing benefits and best-in-class accuracy," said Jared Watkin , senior vice president, Diabetes Care, Abbott. "People living with diabetes are at the center of our design process, and we made our next-generation technology even more discreet for a better user experience to make managing diabetes as easy and seamless as possible."
With a 14-day wear time, the FreeStyle Libre 3 system includes the longest-lasting, 9 self-applied CGM sensor available. The sensor is easy to apply 5 with a one-piece applicator and is worn on the back of the upper arm, eliminating the need for painful fingersticks 10 to view glucose levels.
To allow as many people as possible living with diabetes to access and benefit from the significant advancements of the technology, Abbott's FreeStyle Libre 3 system will be priced the same as previous generations of the device. 7
"Since we launched the first disposable glucose sensor in 2014, we've always believed all people with diabetes should have access to high-quality, accurate and affordable diabetes technologies," said Watkin. "That's why Abbott disrupted the traditional notion that CGMs have to sacrifice quality or accuracy for affordability, and we built our FreeStyle Libre family of products to deliver unparalleled results at a lower cost than any other CGM available."
The FreeStyle Libre 3 system fits seamlessly into the lives of people with diabetes. In addition to the sensor, the system includes the FreeStyle Libre 3 mobile app, 2 which is designed to enable users to capture and view their real-time glucose levels, glucose history and trend arrows showing how their glucose is changing with just a glance at their smartphone.
Abbott designed its next-generation FreeStyle Libre 3 system to be more sustainable for the environment with a smaller and more discreet sensor, reducing the total volume by more than 70%. 11 With a 41% reduction in plastic use and 43% reduction in carton paper, 11 the new sensor design aligns with the company's continued commitment to sustainability.
Abbott is launching the FreeStyle Libre 3 system in Europe in the coming months. The FreeStyle Libre portfolio has been clinically proven to improve glucose control, 12 increase time in target glucose range, 13 decrease time in hyperglycemia (high glucose levels) and hypoglycemia (low glucose levels), 14 and lower HbA1c (average glucose levels over a three-month period) 12 – all factors contributing to better health outcomes. Data also show use of the FreeStyle Libre system reduces diabetes-related hospital admissions and work absentee rates to help improve quality of life. 15
As the #1 sensor-based glucose monitoring system used in the U.S. and worldwide, 8 Abbott's FreeStyle Libre portfolio has changed the lives of more than 2 million people across more than 50 countries 5 by providing breakthrough technology that is accessible and affordable. 7 Abbott has secured partial or full reimbursement for the FreeStyle Libre system in 37 countries, including Canada, France, Germany, Japan, the United Kingdom and the U.S.
About Abbott:
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 107,000 colleagues serve people in more than 160 countries.
Connect with us at www.abbott.com , on LinkedIn at www.linkedin.com/company/abbott-/ , on Facebook at www.facebook.com/Abbott and on Twitter @AbbottNews and @AbbottGlobal.
1 Alva, Shridhara, Timothy Bailey , Ronald Brazg , Erwin S. Budiman , Kristin Castorino , Mark P. Christiansen , Gregory Forlenza , Mark Kipnes , David R. Liljenquist , and Hanqing Liu . "Accuracy of a 14-Day Factory-Calibrated Continuous Glucose Monitoring System With Advanced Algorithm in Pediatric and Adult Population With Diabetes." Journal of Diabetes Science and Technology, ( September 2020 ). https://doi.org/10.1177/1932296820958754 .
2 The FreeStyle Libre 3 app is only compatible with certain mobile devices and operating systems.
3 Among patient-applied sensors. Data on File, Abbott Diabetes Care.
4 Among on-body sensors. Data on File, Abbott Diabetes Care.
5 Data on file. Abbott Diabetes Care.
6 60-minute warm-up required when starting the sensor.
7 Based on a comparison of list prices of the FreeStyle Libre portfolio versus competitor CGM systems available worldwide. The actual cost to patients may or may not be lower than other CGM systems, depending on local reimbursement, if any.
8 Data on file, Abbott Diabetes Care. Data based on the number of users worldwide for the FreeStyle Libre portfolio compared to the number of users for other leading personal use, sensor-based glucose monitoring systems.
9 Among leading CGM brands. Data on File, Abbott Diabetes Care.
10 Finger pricks are required if glucose readings do not match symptoms or expectations.
11 Compared to other FreeStyle Libre systems; Data on file, Abbott Diabetes Care.
12 Eeg-Olofsson et al. Sustainable HbA1c decrease at 12 months for adults with Type 1 and Type 2 Diabetes using the FreeStyle Libre System: a study within the National Diabetes Register in Sweden.
13 Lang J, Jangam SR, Dunn TC, Hayter G. Expanded real-world use confirms strong association between frequency of flash glucose monitoring and glucose control.
14 Dunn TC, Xu Y, Hayter G, Ajjan RA. Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycaemic measures: a European analysis of over 60 million glucose tests. Diabetes Res Clin Pract. 2018;137:37-46.
15 Fokkert M, van Dijk P, Edens M, et al. Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diab Res Care. 2019;7(1):e000809. doi:10.1136/bmjdrc-2019-000809.
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/abbotts-freestyle-libre-3-system-receives-ce-mark--features-worlds-smallest-thinnest-sensor-with-best-in-class-performance-at-the-same-low-cost-for-people-with-diabetes-301138719.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/abbotts-freestyle-libre-3-system-receives-ce-mark--features-worlds-smallest-thinnest-sensor-with-best-in-class-performance-at-the-same-low-cost-for-people-with-diabetes-301138719.html
SOURCE Abbott