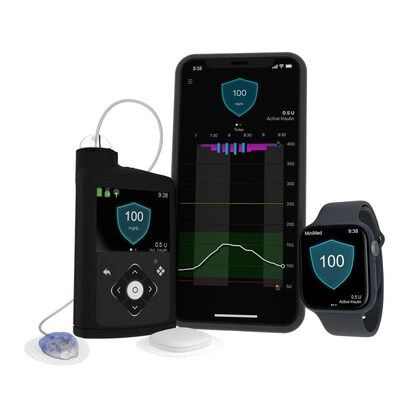
Latest approval expands Medtronic CGM portfolio in the U.S.
Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced the U.S. Food and Drug Administration (FDA) approval for the Simplera Sync™ sensor for use with the MiniMed™ 780G system. With this approval, the MiniMed™ 780G system now offers more flexibility for users of the company's most advanced insulin delivery system featuring Meal Detection™ technology with both the Guardian™ 4 sensor and Simplera Sync™ sensor.
The Simplera Sync™ is a disposable, all-in-one sensor that requires no fingersticks* with SmartGuard™ or overtape and features a simple, two-step insertion process. It is the company's newest addition to its CGM portfolio, which expands options and provides greater flexibility for users.**
The MiniMed™ 780G system's adaptive algorithm automatically anticipates, adjusts, and corrects † glucose levels every 5 minutes, 24/7 – working around the clock so users can focus on what matters. § It's the only system featuring Meal Detection™ technology ‡ , which detects rising sugar levels and delivers more insulin as needed to help users keep glucose levels in range more often – even when users occasionally forget to dose insulin for snacks or meals or underestimate their carbs. The system uses a "treat to target" approach and flexible glucose targets as low as 100 mg/dL, which, combined with its adaptive algorithm allows it to more closely mirror the glucose levels of someone not living with diabetes. Real-world data of the system shows global users consistently achieve time in range above international targets of 70% when using optimal settings (active insulin time of two hours and 100 mg/dL target glucose). 1-3 It is also the only system that works with the world's only infusion set that lasts up to 7 days so that users only have to change their infusion set once per week and can experience 96% fewer injections compared to multiple daily injections.
"We're committed to driving innovation that makes life easier for those living with diabetes so they can forget about their diabetes as much as possible throughout the day," said Que Dallara, EVP and president of Medtronic Diabetes. "Our MiniMed™ 780G system delivers advanced diabetes technology for so many around the world, and we're excited to continue evolving this experience with expanded CGM options —including our Simplera Sync™ sensor, which we look forward to bringing to people living with diabetes in the U.S."
A limited launch of the Simplera Sync™ sensor will begin in the U.S. in the fall of 2025. Today, the MiniMed™ 780G system can be used with the Guardian™ 4 sensor.
About the Diabetes Business at Medtronic ( www.medtronicdiabetes.com )
Medtronic Diabetes is on a mission to alleviate the burden of diabetes by empowering individuals to live life on their terms, with the most advanced diabetes technology and always-on support when and how they need it. We've pioneered first-of-its-kind innovations for over 40 years and are committed to designing the future of diabetes management through next-generation sensors (CGM), intelligent dosing systems, and the power of data science and AI while always putting the customer experience at the forefront.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Galway, Ireland , is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across more than 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE: MDT), visit www.Medtronic.com and follow Medtronic on LinkedIn .
*Fingersticks required in manual mode & to enter SmartGuard. If symptoms don't match alerts & readings, use a fingerstick. Refer to user guide. Pivotal trial participants spend avg of > 93% in SmartGuard.
**Participants in a clinical trial reported the sensor was smaller and lighter than other sensors they have used. Data on file: CIP330, N=243, ages 2-80.
†Refers to auto correct, which provides bolus assistance. Can deliver all correction doses automatically without user interaction, feature can be turned on and off.
‡Taking a bolus 15 – 20 minutes before a meal helps to keep blood sugar levels under control after eating.
§ Refers to SmartGuard™ feature. Individual results may vary.
- Choudhary P. et al, Lancet Diabetes Endocrinol. 2022; https://doi.org/10.1016/ S2213-8587(22)00245-5
- Arrieta A, et al. Diabetes Obes Metab. 2022;10.1111/dom.14714
- Matejko B, et al. Diabetes Care 2022;45:2628–2635
| Contacts: | |
| Ashley Patterson | Ryan Weispfenning |
| Public Relations | Investor Relations |
| +1-818-576-3025 | +1-763-505-4626 |
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/new-simplera-sync-sensor-for-the-minimed-780g-system-now-fda-approved-302432787.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/new-simplera-sync-sensor-for-the-minimed-780g-system-now-fda-approved-302432787.html
SOURCE Medtronic plc