
October 03, 2023
Cleo Diagnostics (ASX:COV) is revolutionising women's healthcare with its disruptive cancer detection platform technology, through a simple blood test that can accurately detect ovarian cancer early. Cleo is well-positioned to leverage this market opportunity considering a compelling addressable market for this technology.
Developed over the course of a decade by Dr. Andrew Stephens, Cleo’s blood test is underpinned by the CXCL10 novel and patented protein biomarker known to be present in all stages of ovarian cancer. By combining CXCL10 with several other biomarkers in a custom algorithm, Cleo can not only be used in triage, but also for the purposes of screening and recurrence testing. The project is currently in the advanced R&D stage and has so far conducted two clinical studies, analysing more than 700 patient samples in the process.
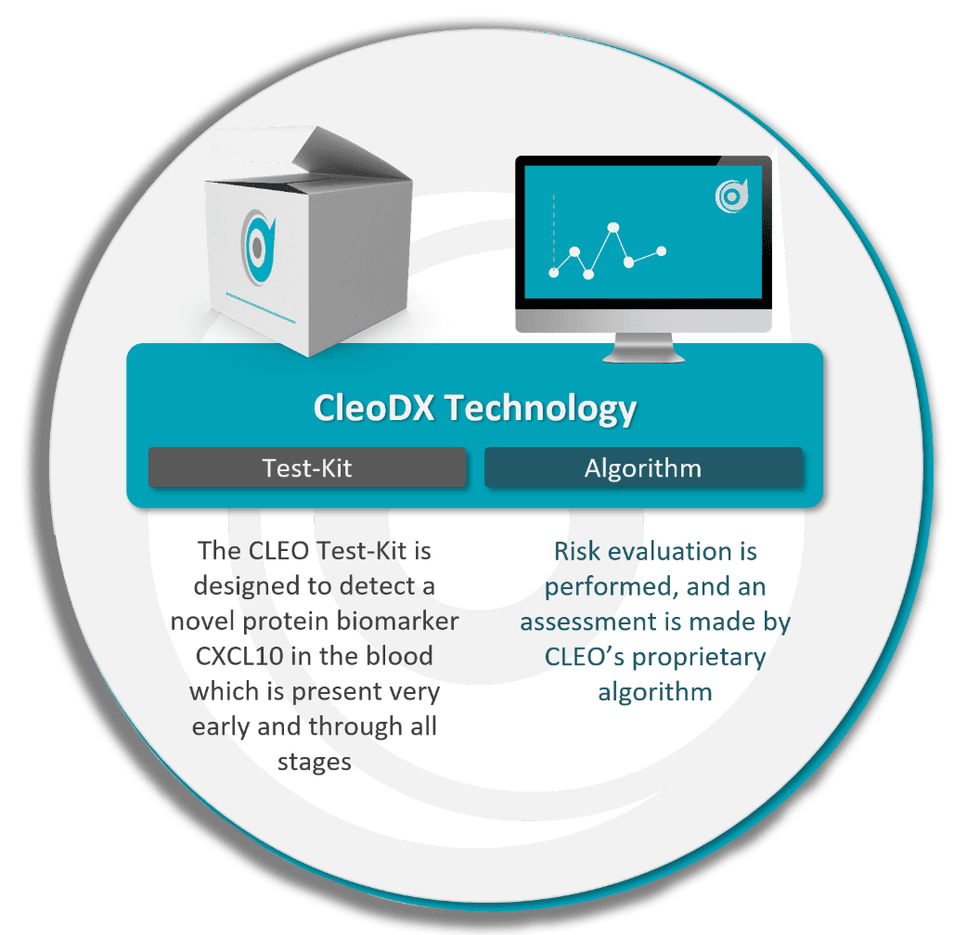
The CleoDX Technology is readily accessible powered with an AI-based risk assessment feature that leverages a proprietary algorithm to perform a risk evaluation on the patient, determining the likelihood of a cancer diagnosis.
Cleo plans for the test to be ready for clinical use in a surgical triage setting by 2025, where it will be available initially to one million patients. Target launch dates for recurrence, high-risk screening and mass screening are still to be determined.
Company Highlights
- Backed by medical professionals and cancer specialists with decades of experience, Cleo Diagnostics has developed a disruptive, accurate and early-stage ovarian cancer detection blood test.
- Cleo targets the CXCL10 novel biomarker, which is now known to be overexpressed in all stages of ovarian cancer.
- Cleo is the result of more than a decade of research at the Hudson Institute of Medical Research, where chief scientist Dr. Andrew Stephens received more than $5 million OCRF & NHMRC funding for development and clinical studies.
- The test is also supported by Professor Tom Jobling, founder of the Ovarian Cancer Foundation and Lucinda Nolan, the foundation's former CEO.
- Cleo has developed a staged execution strategy focused on an achievable path to market. This ensures the project, which is currently in its advanced R&D stage, can maximise commercial value for all stakeholders.
This Cleo Diagnostics profile is part of a paid investor education campaign.*
Click here to connect with Cleo Diagnostics (ASX:COV) to receive an Investor Presentation
COV:AU
The Conversation (0)
21 May 2024
Cleo Diagnostics
Revolutionising Ovarian Cancer Diagnosis Through Accurate and Early Detection
Revolutionising Ovarian Cancer Diagnosis Through Accurate and Early Detection Keep Reading...
17 March 2025
CLEO Delivers Commercial Development Milestone
Cleo Diagnostics (COV:AU) has announced CLEO Delivers Commercial Development MilestoneDownload the PDF here. Keep Reading...
27 February 2025
Appendix 4D and Half Yearly Accounts
Cleo Diagnostics (COV:AU) has announced Appendix 4D and Half Yearly AccountsDownload the PDF here. Keep Reading...
31 January 2025
Quarterly activities and cashflow report
Cleo Diagnostics (COV:AU) has announced Quarterly activities and cashflow reportDownload the PDF here. Keep Reading...
09 December 2024
CLEO Further Expands Ovarian Cancer Trial with Siles Health
Cleo Diagnostics (COV:AU) has announced CLEO Further Expands Ovarian Cancer Trial with Siles HealthDownload the PDF here. Keep Reading...
26 November 2024
The Royal Women's Hospital Joins CLEO Ovarian Cancer Trial
Cleo Diagnostics (COV:AU) has announced The Royal Women's Hospital Joins CLEO Ovarian Cancer TrialDownload the PDF here. Keep Reading...
28 January
Seegnal Presents Real-World Evidence on Reducing Fall Risk in Geriatric Patients at Caltcm Summit
Seegnal Inc. (TSXV: SEGN) ("Seegnal" or the "Corporation"), a global leader in AI-enhanced prescription intelligence, today announced real-world clinical results demonstrating how medication governance may reduce fall-risk drivers in older adults -- a significant clinical and financial challenge... Keep Reading...
05 January
Pathways to Commercialising Biotech Innovations
In the medical technology industry, innovation is only the first step. While key to long-term success, innovation is only as good as a company’s commercialisation strategy. Once a technology has been developed and proven, the organisation must then embark on a process to commercialise it for... Keep Reading...
25 February 2025
HeraMED Signs Strategic Collaboration Agreement with Garmin Health
HeraMED Limited (ASX: HMD), a medical data and technology company leading the digital transformation of maternity care, is delighted to announce it has entered into a collaboration agreement with Garmin (NYSE: GRMN), a leading global provider of smartwatches and GPS-enabled products, aimed at... Keep Reading...
23 January 2025
Cyclopharm Signs US Agreement with HCA Healthcare for Technegas®
Cyclopharm Limited (ASX: CYC) is pleased to announce the signing of a major contract with Hospital Corporation of America Healthcare (HCA), one of the largest single healthcare providers in the United States. This agreement marks a significant milestone for the company which will allow the... Keep Reading...
23 January 2025
CONNEQT App Launches in USA as Pulse Deliveries Commence
Cardiex Limited (CDX:AU) has announced CONNEQT App Launches in USA as Pulse Deliveries CommenceDownload the PDF here. Keep Reading...
16 January 2025
Revolutionizing Women's Health: Antifungal Innovation Brings New Investment Opportunities
The intersection of women's health and antifungal innovation represents a pivotal moment in healthcare, offering both transformative medical advancements and compelling investment opportunities. The groundbreaking developments in antifungal treatments specifically targeting women's health issues... Keep Reading...
Latest News
Interactive Chart
Latest Press Releases
Related News
TOP STOCKS
American Battery4.030.24
Aion Therapeutic0.10-0.01
Cybin Corp2.140.00









