Continued enrollment globally in multiple clinical studies of vepdegestrant in ER+HER2- metastatic breast cancer, including the VERITAC-2 Phase 3 trial in the second-line setting and the study lead-in for the VERITAC-3 Phase 3 trial in the first-line setting –
– Top-line data readout for VERITAC-2 remains on-track for 2H 2024 –
– Entered a transaction with Novartis providing an exclusive license for ARV-766 and sale of preclinical AR-V7 program; $150 million upfront payment and potential for up to $1.01 billion in milestones and royalties under license agreement –
– Initiated dosing in a first-in-human Phase 1 clinical trial with ARV-102, the first oral PROTAC® degrader in clinical development to treat neurodegenerative diseases –
NEW HAVEN, Conn., May 07, 2024 (GLOBE NEWSWIRE) -- Arvinas, Inc. (Nasdaq: ARVN), a clinical-stage biotechnology company creating a new class of drugs based on targeted protein degradation, today reported financial results for the first quarter ended March 31, 2024 and provided a corporate update.
"Our recently announced agreement with Novartis creates significant value for Arvinas and further validates our innovative PROTAC protein degrader platform and its potential to deliver important new treatment options for patients," said John Houston, Ph.D., Chairperson, President and Chief Executive Officer at Arvinas. "In addition to this strategic transaction, and together with Pfizer, we continued making meaningful progress advancing our Phase 3 clinical programs with vepdegestrant in ER+/HER2- metastatic breast cancer. During the quarter we also advanced ARV-102, our first PROTAC degrader with the potential to treat neurodegenerative diseases, into a Phase 1 clinical trial and we remain on track to initiate a first-in-human Phase 1 clinical trial with ARV-393, our BCL6 targeting PROTAC degrader, by the end of the second quarter. We also further strengthened our management team with key hires as we approach our first Phase 3 readout and continue progressing multiple ongoing and planned clinical-stage programs."
Recent Developments and First Quarter Business Highlights
Strategic Transaction with Novartis
- Announced an exclusive strategic license agreement with Novartis (NYSE: NVS) for the worldwide development and commercialization of ARV-766, Arvinas' second generation PROTAC® androgen receptor (AR) degrader for patients with prostate cancer, and the sale of Arvinas' preclinical AR-V7 program.
- Upon closing, Arvinas will receive a $150 million upfront payment for the license of ARV-766 and the sale of Arvinas' preclinical AR-V7 program, with the potential under the License Agreement for up to $1.01 billion in development, regulatory, and commercial milestones, as well as tiered royalties.
Vepdegestrant
- Completed enrollment of the study lead-in for the VERITAC-3 Phase 3 clinical trial of vepdegestrant and palbociclib as a first-line treatment in patients with estrogen receptor (ER) positive/human growth epidermal growth factor 2 (HER2) negative (ER+/HER2-) locally advanced or metastatic breast cancer.
- Received U.S. Food and Drug Administration Fast Track designation for the investigation of vepdegestrant for monotherapy in the treatment of adults with ER+/HER2- locally advanced or metastatic breast cancer previously treated with endocrine-based therapy.
- Initiated dosing in a Phase 1b/2 clinical trial with vepdegestrant plus Pfizer's novel CDK4 inhibitor atirmociclib (PF-07220060) (TACTIVE-K: ClinicalTrials.gov Identifier: NCT06206837).
- Initiated dosing in an additional arm of the Phase 1b/2 combination umbrella trial with the CDK7 inhibitor samuraciclib (TACTIVE-U: ClinicalTrials.gov Identifiers: NCT05548127, NCT05573555, and NCT06125522).
- Announced the inclusion of an additional arm in the I-SPY-2 Endocrine Optimization Platform (EOP) study (NCT01042379) that will evaluate vepdegestrant in combination with abemaciclib.
- Vepdegestrant is also being evaluated in a monotherapy arm and in combination with letrozole arm in the ongoing I-SPY TRIAL endocrine optimization program sponsored by Quantum Leap.
Pipeline
- Initiated dosing in a first-in-human Phase 1 clinical trial in healthy volunteers with ARV-102, the Company's first neuroscience PROTAC degrader targeting leucine-rich repeat kinase 2 (LRRK2) as a potential treatment for idiopathic Parkinson's disease and progressive supranuclear palsy.
Corporate
- Announced the appointment of Noah Berkowitz, M.D, Ph.D., to the role of Chief Medical Officer.
- Announced the appointment of Jared Freedberg, J.D., as General Counsel.
- Announced the resignation of Chief Financial Officer and Treasurer, Sean Cassidy, effective February 29, 2024.
- Announced the appointment of Randy Teel, Ph.D., Arvinas' current Senior Vice President of Corporate and Business Development and Interim Chief Financial Officer and Treasurer, to the newly created position of Chief Business Officer.
- Dr. Teel will remain in his interim roles while the Arvinas board of directors continues its search for a permanent Chief Financial Officer and Treasurer.
- Dr. Teel will remain in his interim roles while the Arvinas board of directors continues its search for a permanent Chief Financial Officer and Treasurer.
Anticipated Upcoming Milestones and Expectations
Vepdegestrant
As part of Arvinas' global collaboration with Pfizer, the companies plan to:
- Complete enrollment and announce topline data for the VERITAC-2 Phase 3 monotherapy trial (ClinicalTrials.gov Identifier: NCT05654623) in patients with metastatic breast cancer (2H 2024).
- Determine the recommended Phase 3 dose of palbociclib to be administered in combination with vepdegestrant from the study-lead in of the VERITAC-3 Phase 3 trial of vepdegestrant and palbociclib as a first-line treatment in patients with ER+/HER2- locally advanced or metastatic breast cancer (2H 2024).
- Continue enrollment of the ongoing Phase 1b/2 clinical trial with vepdegestrant plus Pfizer's novel CDK4 inhibitor atirmociclib (TACTIVE-K: ClinicalTrials.gov Identifier: NCT06206837).
- Continue enrollment of the ongoing Phase 1b combination umbrella trial evaluating combinations of vepdegestrant with abemaciclib, ribociclib, or samuraciclib (TACTIVE-U: ClinicalTrials.gov Identifiers: NCTC05548127, NCTC05573555, and NCT06125522).
- Initiate discussion with regulatory authorities on a second-line Phase 3 trial of vepdegestrant in combination with palbociclib and potentially other CDK4/6 inhibitors, and a new first-line Phase 3 trial of vepdegestrant plus atirmociclib, Pfizer's novel CDK4 inhibitor.
ARV-766
- Following US antitrust regulatory review, currently expected to conclude in Q2 2024, initiate exclusive strategic license agreement with Novartis for the worldwide development and commercialization of ARV-766 and asset purchase agreement for the sale of Arvinas' preclinical AR-V7 program.
Pipeline
- Continue enrollment in Phase 1 clinical trial in healthy volunteers with PROTAC LRRK2 degrader ARV-102.
- Initiate dosing in first-in-human Phase 1 clinical trial in B-cell lymphomas with PROTAC BCL6 degrader ARV-393 (2Q 2024).
Financial Guidance
Based on its current operating plan, Arvinas believes its cash, cash equivalents, restricted cash and marketable securities as of March 31, 2024, is sufficient to fund planned operating expenses and capital expenditure requirements into 2027.
First Quarter Financial Results
Cash, Cash Equivalents and Marketable Securities Position : As of March 31, 2024, cash, cash equivalents, restricted cash and marketable securities were $1,174.8 million as compared with $1,266.5 million as of December 31, 2023. The decrease in cash, cash equivalents, restricted cash and marketable securities of $91.7 million for the three months ended March 31, 2024, was primarily related to cash used in operations of $92.1 million, unrealized losses on marketable securities of $1.3 million and leasehold improvements of $0.1 million, partially offset by proceeds from the exercise of stock options of $1.8 million.
Research and Development Expenses: Research and development expenses were $84.3 million for the quarter ended March 31, 2024, as compared with $95.3 million for the quarter ended March 31, 2023. The decrease in research and development expenses of $11.0 million for the quarter was primarily due to a decrease in expenses related to our AR program (which includes ARV-766 and bavdegalutamide (ARV-110)) of $8.2 million, our ER program (which includes the cost sharing of vepdegestrant under the Vepdegestrant (ARV-471) Collaboration Agreement) of $2.3 million and our platform and exploratory programs of $0.5 million.
General and Administrative Expenses: General and administrative expenses were $24.3 million for the quarter ended March 31, 2024, as compared with $24.9 million for the quarter ended March 31, 2023. The decrease of $0.6 million was primarily due to a decrease in personnel and infrastructure related costs of $2.4 million, partially offset by an increase in professional fees of $1.3 million and increases related to establishing our commercial operations of $0.6 million.
Revenues: Revenues were $25.3 million for the quarter ended March 31, 2024 as compared with $32.5 million for the quarter ended March 31, 2023. Revenue is related to the Vepdegestrant (ARV-471) Collaboration Agreement, the collaboration and license agreement with Bayer, the collaboration and license agreement with Pfizer, the amended and restated option, license and collaboration agreement with Genentech and revenue related to our Oerth Bio joint venture. The decrease in revenue of $7.2 million was primarily due to a decrease in revenue from the Vepdegestrant (ARV-471) Collaboration Agreement of $12.5 million, a decrease of $1.8 million related to the conclusion of the performance period under the collaboration agreement with Genentech and a decrease of $1.1 million of previously constrained deferred revenue related to our Oerth Bio joint venture, offset in part by year over year increases in revenue of $5.5 million and $2.6 million from our collaboration and license agreements with Bayer and Pfizer, respectively, due to changes in estimates in 2023 of the performance period duration resulting from updated research timelines.
About Vepdegestrant (ARV-471)
Vepdegestrant is an investigational, orally bioavailable PROTAC protein degrader designed to specifically target and degrade the estrogen receptor (ER) for the treatment of patients with ER positive (ER+)/human epidermal growth factor receptor 2 (HER2) negative (ER+/HER2-) breast cancer. Vepdegestrant is being developed as a potential monotherapy and as part of combination therapy across multiple treatment settings for ER+/HER2- metastatic breast cancer.
In July 2021, Arvinas announced a global collaboration with Pfizer for the co-development and co-commercialization of vepdegestrant; Arvinas and Pfizer will share worldwide development costs, commercialization expenses, and profits.
Vepdegestrant has been granted Fast Track designation by the U.S. Food and Drug Administration (FDA) for the investigation of vepdegestrant for monotherapy in the treatment of adults with ER+/HER2- locally advanced or metastatic breast cancer previously treated with endocrine-based therapy.
About ARV-766
ARV-766 is an investigational orally bioavailable PROTAC protein degrader designed to selectively target and degrade the androgen receptor (AR). Preclinically, ARV-766 has demonstrated activity in models of wild type androgen receptor tumors in addition to tumors with AR mutations or amplification, both common potential mechanisms of resistance to currently available AR-targeted therapies.
About Arvinas
Arvinas is a clinical-stage biotechnology company dedicated to improving the lives of patients suffering from debilitating and life-threatening diseases through the discovery, development, and commercialization of therapies that degrade disease-causing proteins. Arvinas uses its proprietary PROTAC Discovery Engine platform to engineer proteolysis targeting chimeras, or PROTAC targeted protein degraders, that are designed to harness the body's own natural protein disposal system to selectively and efficiently degrade and remove disease-causing proteins. In addition to its robust preclinical pipeline of PROTAC protein degraders against validated and "undruggable" targets, the company has four investigational clinical-stage programs: vepdegestrant (ARV-471) for the treatment of patients with locally advanced or metastatic ER+/HER2- breast cancer; ARV-766 and bavdegalutamide for the treatment of patients with metastatic castration-resistant prostate cancer; and ARV-102 for the treatment of patients with neurodegenerative disorders. For more information, visit www.arvinas.com .
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties, including statements regarding: the expected timing in connection with the completion of enrollment and readout of top-line data from the VERITAC-2 clinical trial; the expected timing of the initiation of a first-in-human Phase 1 clinical trial with ARV-393; the potential of Arvinas' PROTAC protein degrader platform and its potential to deliver new treatment options to patients; Arvinas' and Pfizer, Inc.'s ("Pfizer") plans to determine the recommended Phase 3 dose of palbociclib to be administered in combination with vepdegestrant from the study-lead in of the VERITAC-3 Phase 3 trial of vepdegestrant and palbociclib; Arvinas' and Pfizer's plans to initiate a discussion with regulatory authorities on a second-line Phase 3 trial of vepdegestrant in combination with palbociclib and potentially other CDK4/6 inhibitors, and a new first-line Phase 3 trial of vepdegestrant plus atirmociclib; the closing of the transaction with Novartis and the receipt of upfront, milestone, and royalty payments in connection with the transaction and the future development, potential marketing approval and commercialization of ARV-766; and statements regarding Arvinas' cash, cash equivalents, restricted cash and marketable securities. All statements, other than statements of historical fact, contained in this press release, including statements regarding Arvinas' strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words "anticipate," "believe," "estimate," "expect," "intend," "may," "might," "plan," "predict," "project," "target," "potential," "will," "would," "could," "should," "continue," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
Arvinas may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on such forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements Arvinas makes as a result of various risks and uncertainties, including but not limited to: Arvinas' and Pfizer's performance of the respective obligations with respect to Arvinas' collaboration with Pfizer; whether Arvinas and Pfizer will be able to successfully conduct and complete clinical development for vepdegestrant; whether Arvinas will be able to successfully conduct and complete development for its other product candidates, including ARV-766, and including whether Arvinas initiates and completes clinical trials for its product candidates and receive results from its clinical trials on its expected timelines or at all; whether Arvinas and Pfizer, as appropriate, will be able to obtain marketing approval for and commercialize vepdegestrant, ARV-766 and other product candidates on current timelines or at all; the satisfaction or waiver of the closing conditions set forth in the license agreement with Novartis, each party's performance of its obligations under the license agreement; whether Novartis will be able to successfully conduct and complete clinical development, obtain marketing approval for and commercialize ARV-766; Arvinas' ability to protect its intellectual property portfolio; whether Arvinas' cash and cash equivalent resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements; and other important factors discussed in the "Risk Factors" section of Arvinas' Annual Report on Form 10-K for the year ended December 31, 2023 and subsequent other reports on file with the U.S. Securities and Exchange Commission. The forward-looking statements contained in this press release reflect Arvinas' current views with respect to future events, and Arvinas assumes no obligation to update any forward-looking statements, except as required by applicable law. These forward-looking statements should not be relied upon as representing Arvinas' views as of any date subsequent to the date of this release.
Contacts
Investors:
Jeff Boyle
+1 (347) 247-5089
Jeff.Boyle@arvinas.com
Media:
Kathleen Murphy
+1 (760) 622-3771
Kathleen.Murphy@arvinas.com
| Arvinas, Inc. | |||||||
| Condensed Consolidated Balance Sheets (Unaudited) | |||||||
| (dollars and shares in millions, except per share amounts) | March 31, 2024 | December 31, 2023 | |||||
| Assets | |||||||
| Current assets: | |||||||
| Cash and cash equivalents | $ | 88.0 | $ | 311.7 | |||
| Restricted cash | 5.5 | 5.5 | |||||
| Marketable securities | 1,081.3 | 949.3 | |||||
| Other receivables | 7.1 | 7.2 | |||||
| Prepaid expenses and other current assets | 8.4 | 6.5 | |||||
| Total current assets | 1,190.3 | 1,280.2 | |||||
| Property, equipment and leasehold improvements, net | 10.4 | 11.5 | |||||
| Operating lease right of use assets | 2.0 | 2.5 | |||||
| Collaboration contract asset and other assets | 9.9 | 10.4 | |||||
| Total assets | $ | 1,212.6 | $ | 1,304.6 | |||
| Liabilities and stockholders' equity | |||||||
| Current liabilities: | |||||||
| Accounts payable and accrued liabilities | $ | 76.4 | $ | 92.2 | |||
| Deferred revenue | 162.9 | 163.0 | |||||
| Current portion of operating lease liabilities | 1.5 | 1.9 | |||||
| Total current liabilities | 240.8 | 257.1 | |||||
| Deferred revenue | 361.0 | 386.2 | |||||
| Long term debt | 0.7 | 0.8 | |||||
| Operating lease liabilities | 0.4 | 0.5 | |||||
| Total liabilities | 602.9 | 644.6 | |||||
| Stockholders' equity: | |||||||
| Preferred stock, $0.001 par value, zero shares issued and outstanding as of March 31, 2024 and December 31, 2023, respectively | — | — | |||||
| Common stock, $0.001 par value; 68.3 and 68.0 shares issued and outstanding as of March 31, 2024 and December 31, 2023, respectively | 0.1 | 0.1 | |||||
| Accumulated deficit | (1,402.1 | ) | (1,332.7 | ) | |||
| Additional paid-in capital | 2,016.1 | 1,995.7 | |||||
| Accumulated other comprehensive loss | (4.4 | ) | (3.1 | ) | |||
| Total stockholders' equity | 609.7 | 660.0 | |||||
| Total liabilities and stockholders' equity | $ | 1,212.6 | $ | 1,304.6 | |||
| Arvinas, Inc. | |||||||
| Condensed Consolidated Statements of Operations (Unaudited) | |||||||
| For the Three Months Ended March 31, | |||||||
| (dollars and shares in millions, except per share amounts) | 2024 | 2023 | |||||
| Revenue | $ | 25.3 | $ | 32.5 | |||
| Operating expenses: | |||||||
| Research and development | 84.3 | 95.3 | |||||
| General and administrative | 24.3 | 24.9 | |||||
| Total operating expenses | 108.6 | 120.2 | |||||
| Loss from operations | (83.3 | ) | (87.7 | ) | |||
| Interest and other income | 14.0 | 6.5 | |||||
| Net loss before income taxes and loss from equity method investment | (69.3 | ) | (81.2 | ) | |||
| Income tax (expense) benefit | (0.1 | ) | 0.4 | ||||
| Loss from equity method investment | — | (1.1 | ) | ||||
| Net loss | $ | (69.4 | ) | $ | (81.9 | ) | |
| Net loss per common share, basic and diluted | $ | (0.97 | ) | $ | (1.54 | ) | |
| Weighted average common shares outstanding, basic and diluted | 71.7 | 53.3 | |||||


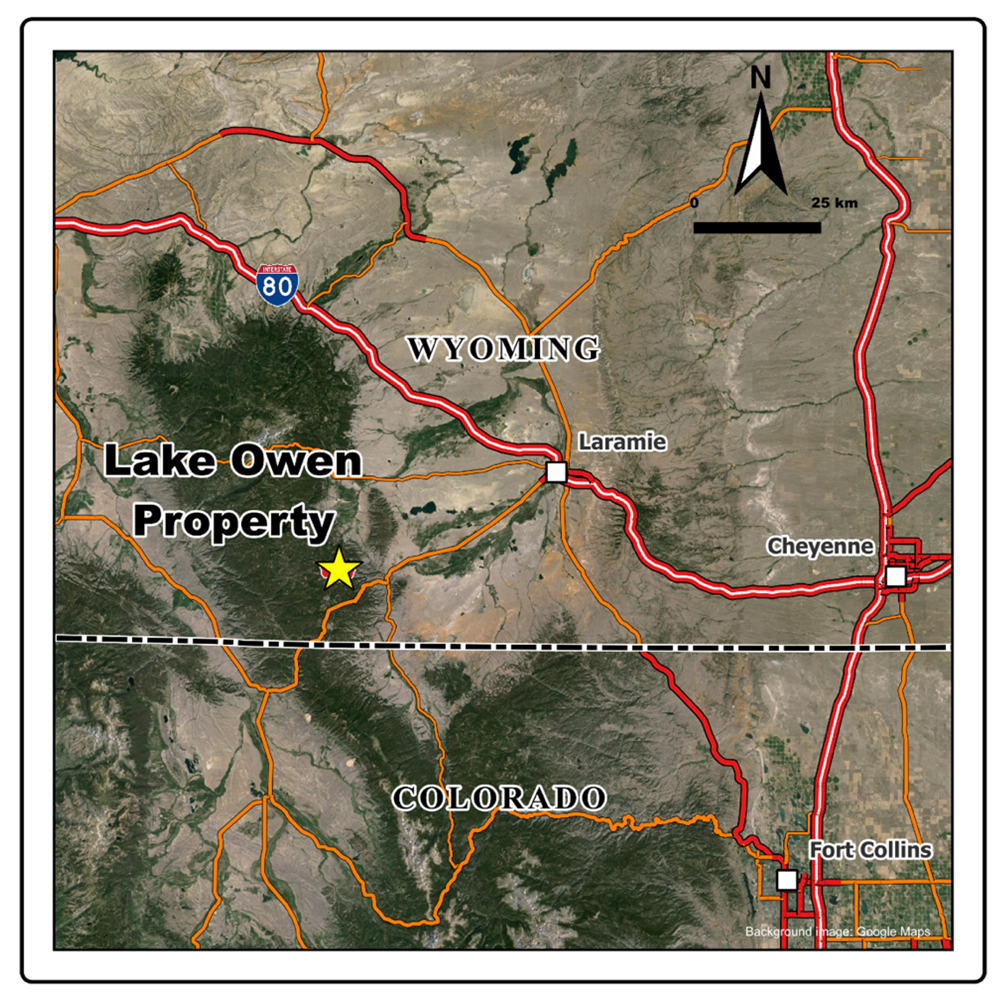 Figure 1 - Location Map, Lake Owen Project, Wyoming, USA
Figure 1 - Location Map, Lake Owen Project, Wyoming, USA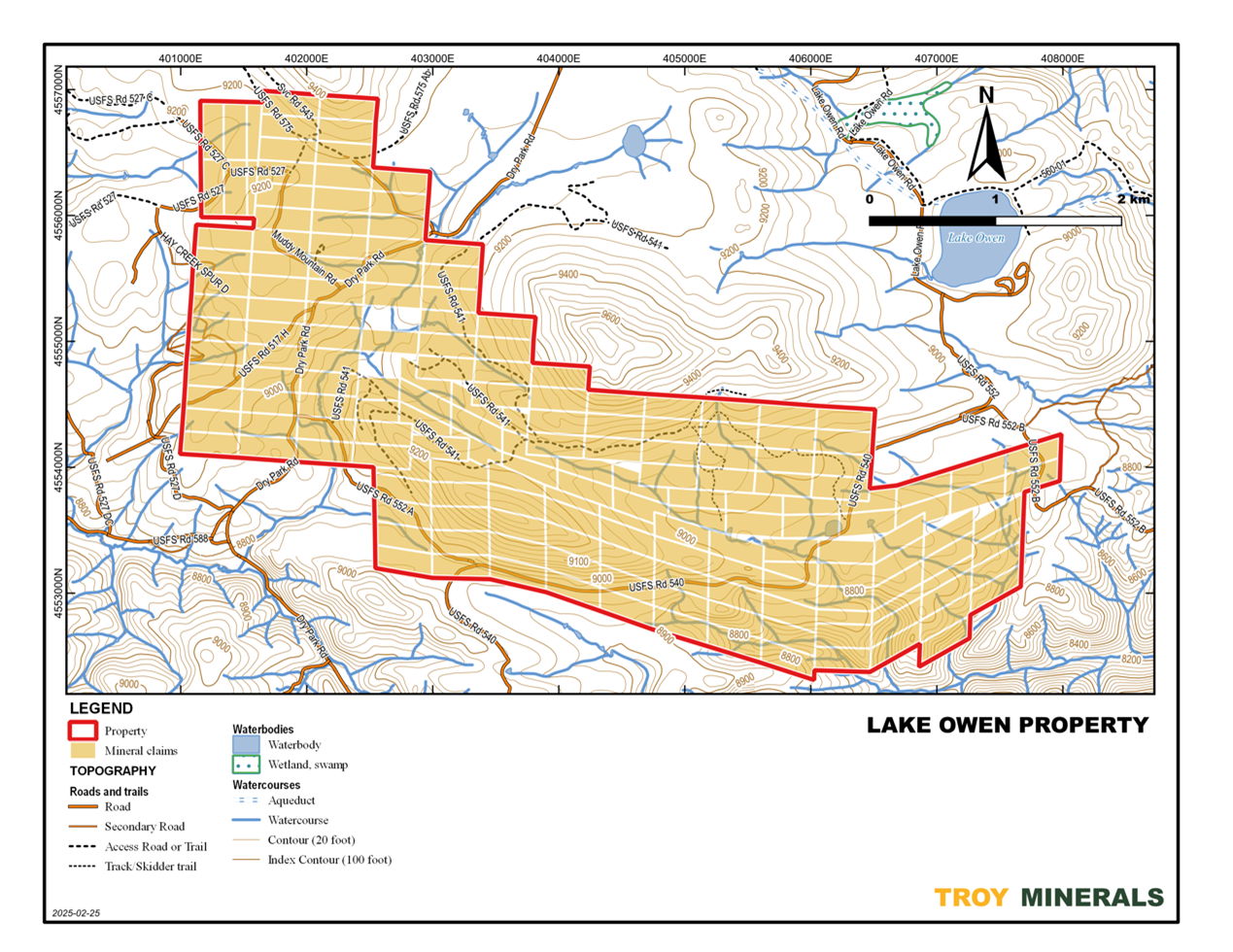 Figure 2 - Claim Map, Lake Owen Project
Figure 2 - Claim Map, Lake Owen Project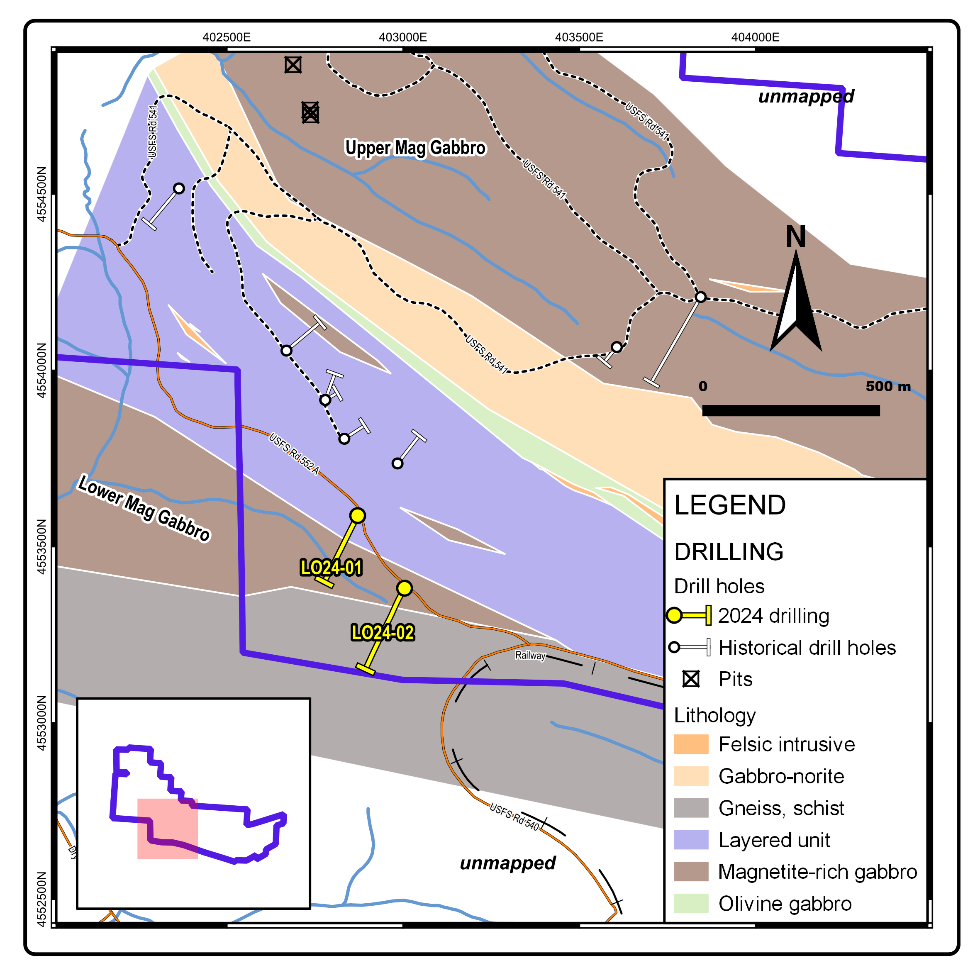 Figure 3 - Drill Hole Locations
Figure 3 - Drill Hole Locations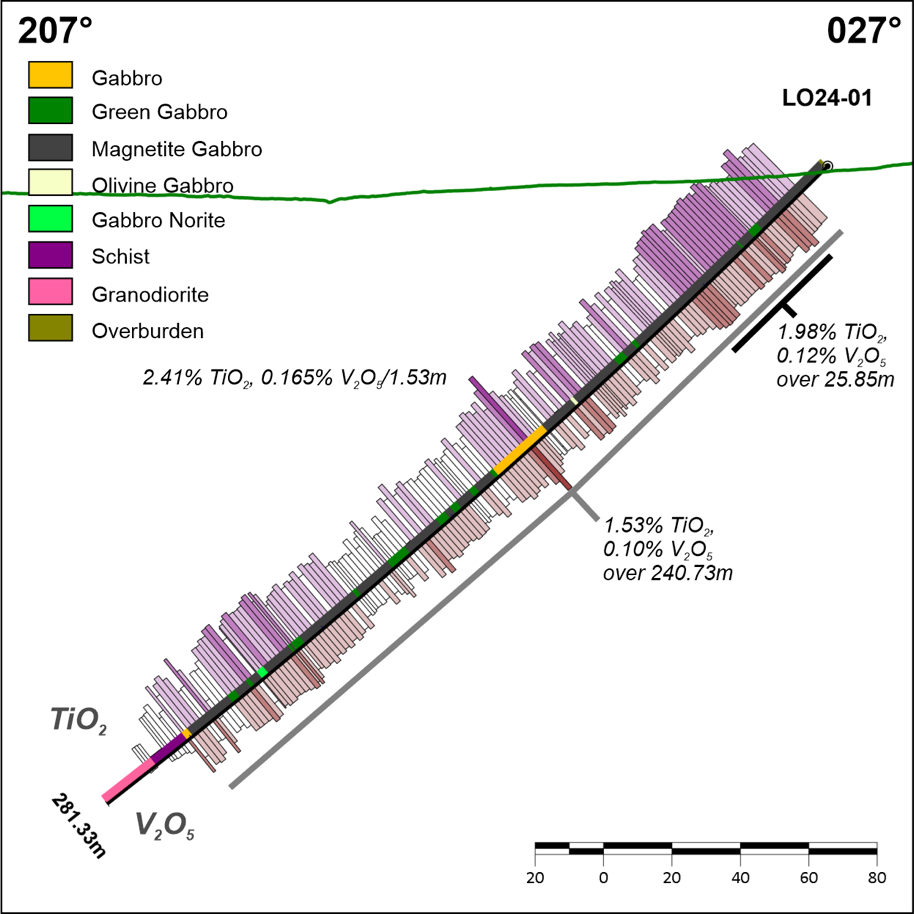 Figure 4 - DDH Cross Section, LO24-01
Figure 4 - DDH Cross Section, LO24-01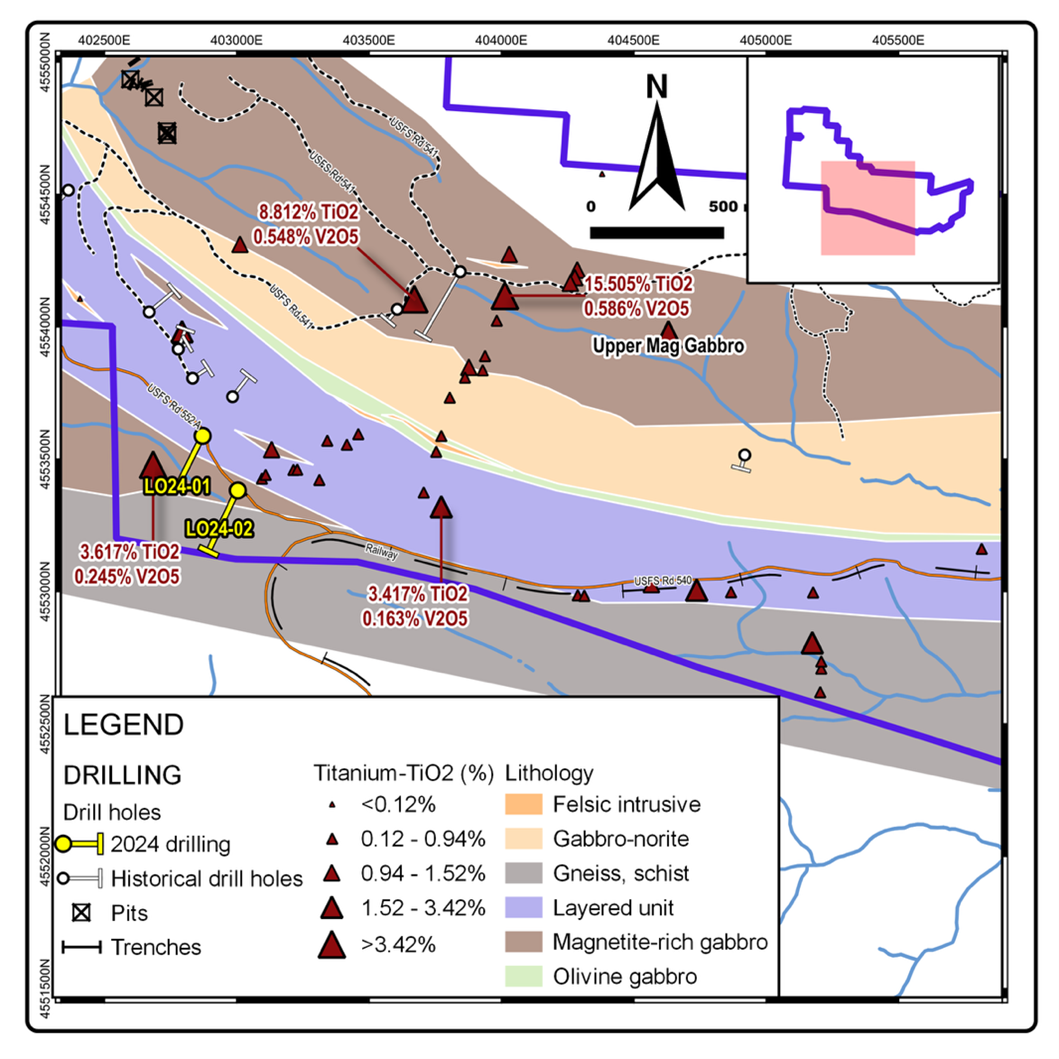 Figure 5 - Surface Rock Sampling - Titanium Values (part of the Project)
Figure 5 - Surface Rock Sampling - Titanium Values (part of the Project)











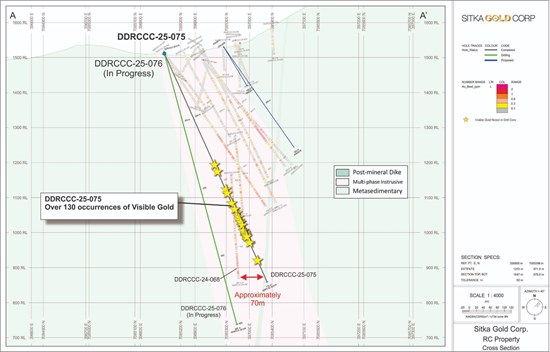



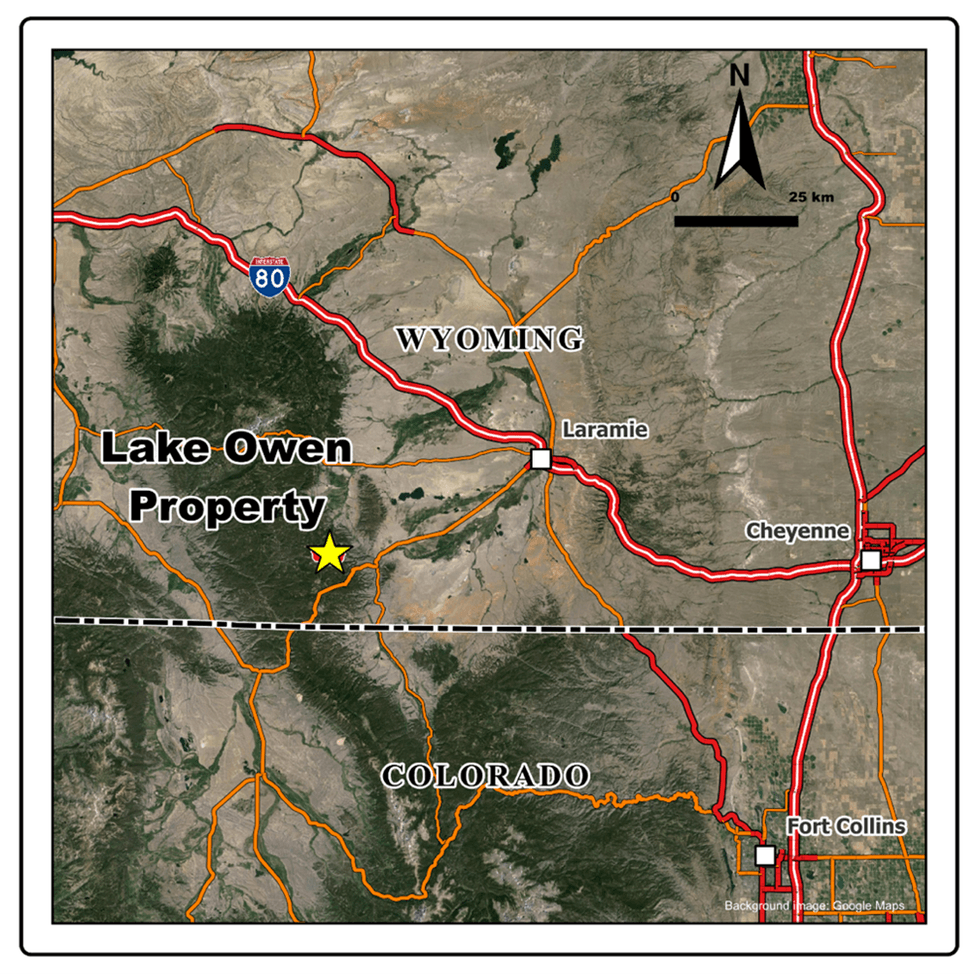 Figure 1. Lake Owen, Location Map, Wyoming, USA
Figure 1. Lake Owen, Location Map, Wyoming, USA 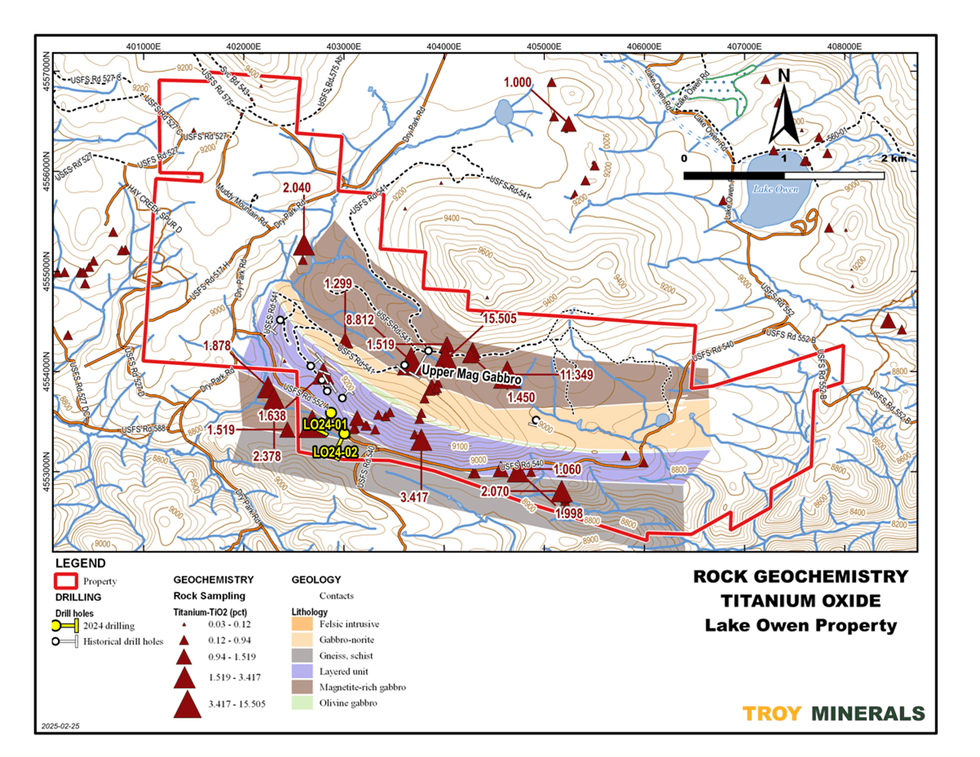 Figure 2. Lake Own Project, Troy's Claims on Topo Map
Figure 2. Lake Own Project, Troy's Claims on Topo Map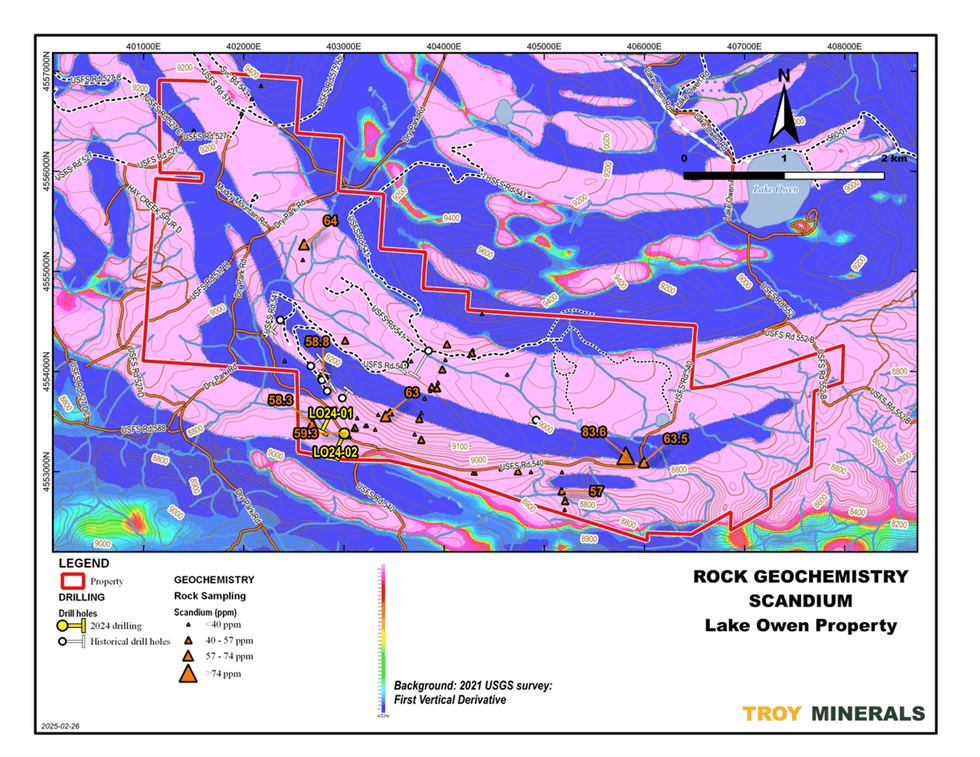 Figure 3. Drill Hole Locations on top of Geology and Company's Claims
Figure 3. Drill Hole Locations on top of Geology and Company's Claims 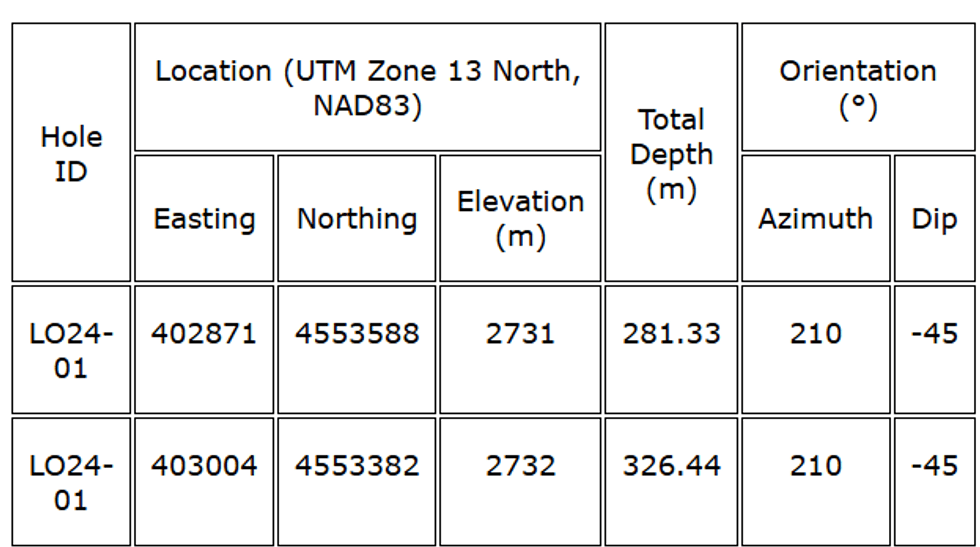 Table 1: Drill hole specifications
Table 1: Drill hole specifications 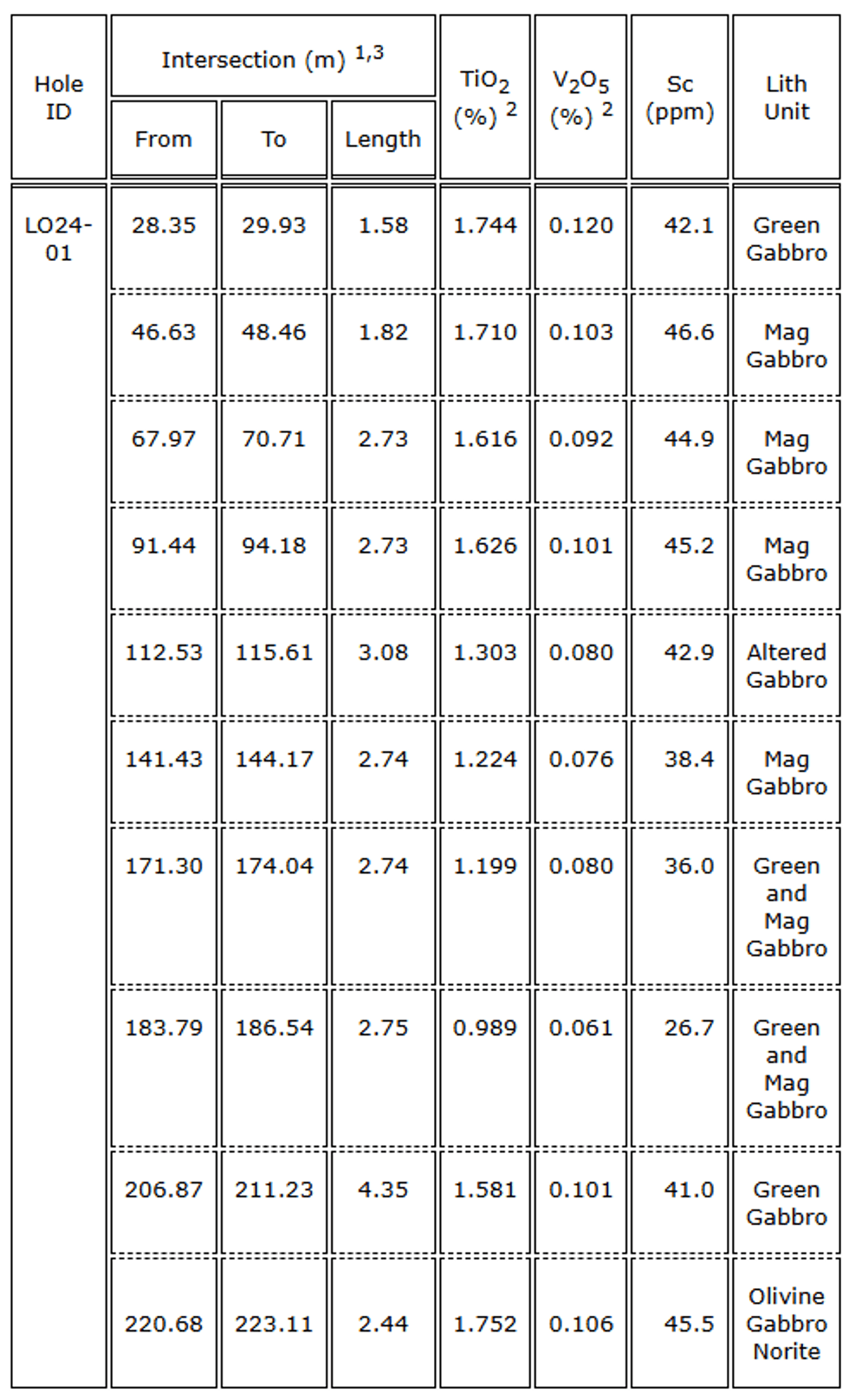 Table 2. Drill hole LO24-01 intersections
Table 2. Drill hole LO24-01 intersections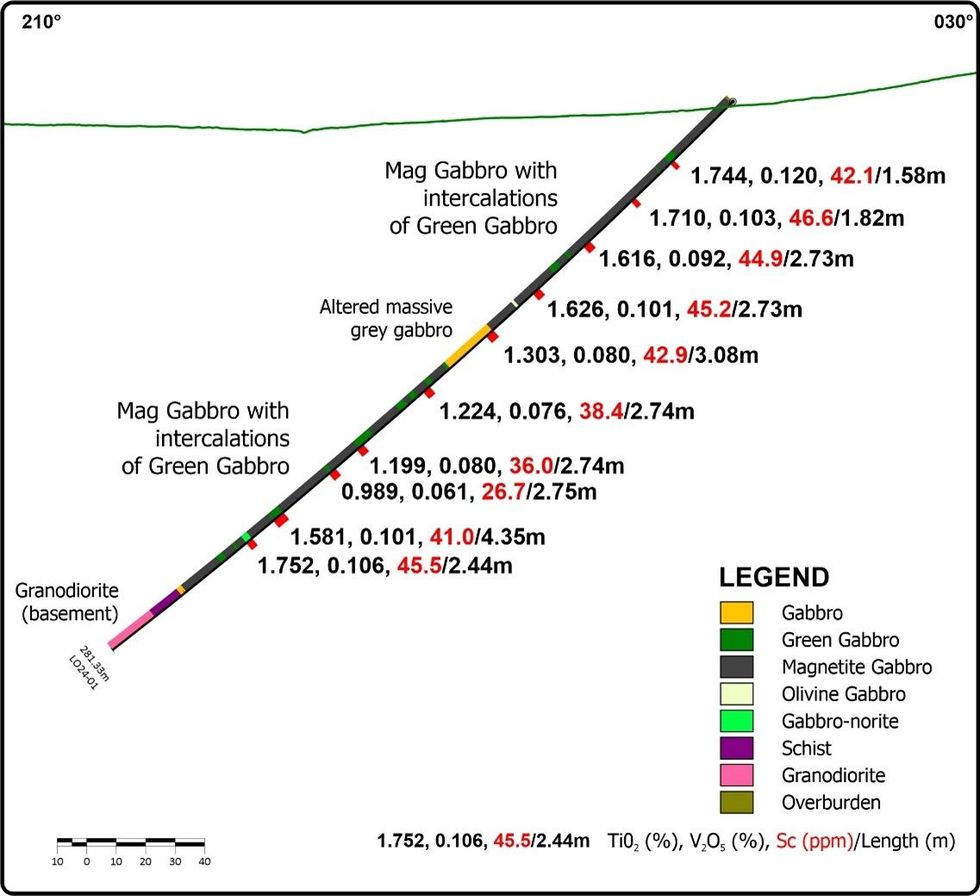 Figure 4. DDH Cross Section, LO24-01
Figure 4. DDH Cross Section, LO24-01 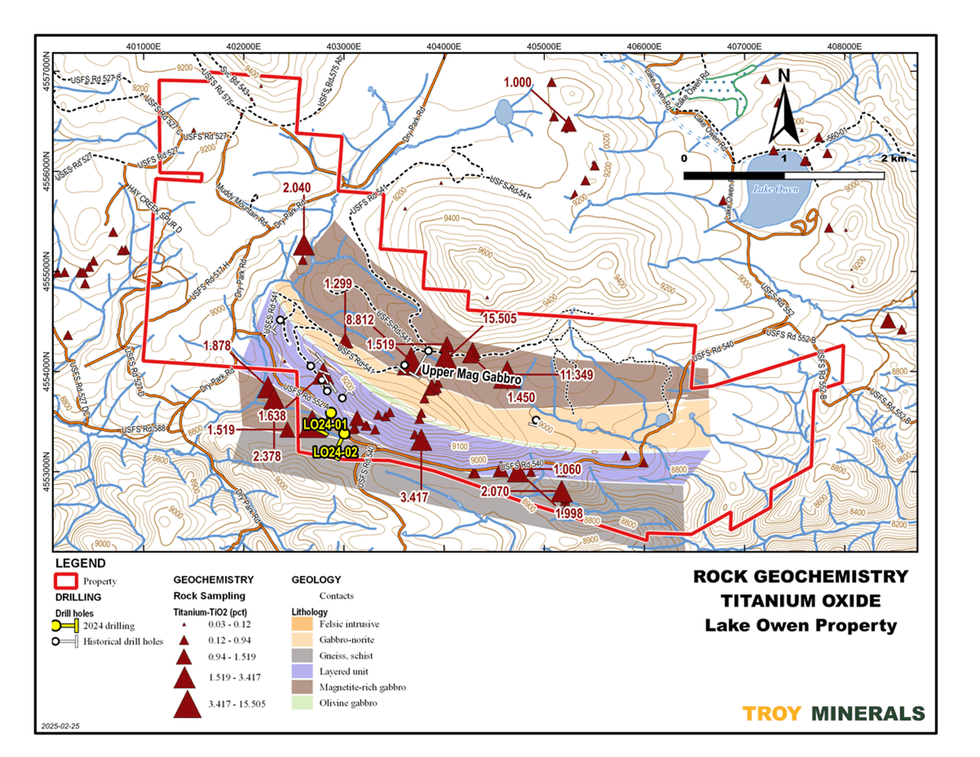 Figure 5. Rock Geochemistry, Titanium
Figure 5. Rock Geochemistry, Titanium 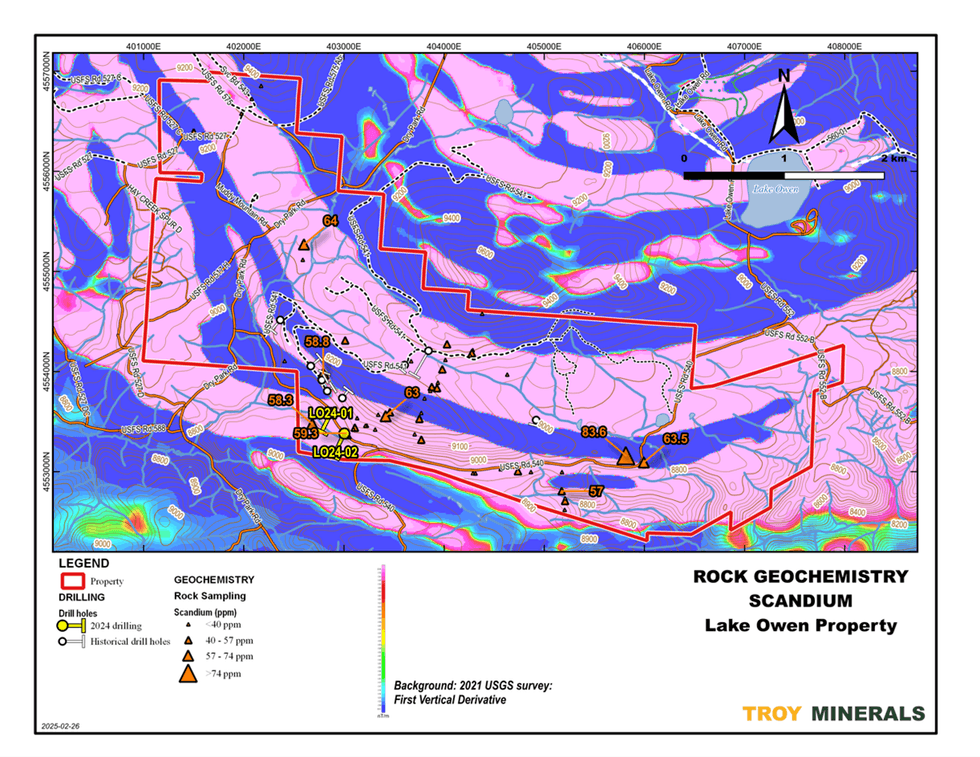 Figure 6. Rock Geochemistry, Scandium on top of Airborne Magnetics (1st Vertical Derivative)
Figure 6. Rock Geochemistry, Scandium on top of Airborne Magnetics (1st Vertical Derivative)