Publication title: "Oral Blarcamesine Phase IIbIII Trial Confirms Identified Precision Medicine Patient Population Significant Broad Clinical and Quality of Life Improvements for Early Alzheimer's Disease Patients"
Significant improvement in self-assessed Quality of Life (QoL-AD) scores indicating a reversal of negative trajectory for Alzheimer's disease clinical trial participants was observed
Using a novel Precision Medicine approach, up to ~70% of Alzheimer's disease participants benefited with unprecedented effect size
Oral blarcamesine tablet, by enhancing the brain's autophagy internal clearing mechanism could alleviate significant medical and economic burden
Publication available on medRxiv, in submission to a peer-reviewed medical journal
NEW YORK, Sept. 30, 2025 (GLOBE NEWSWIRE) -- Anavex Life Sciences Corp. ("Anavex" or the "Company") (Nasdaq: AVXL), a clinical-stage biopharmaceutical company focused on developing innovative treatments for Alzheimer's disease, Parkinson's disease, schizophrenia, neurodevelopmental, neurodegenerative, and rare diseases, including Rett syndrome, and other central nervous system (CNS) disorders, today announced the publication " Oral Blarcamesine Phase IIb/III Trial Confirms Identified Precision Medicine Patient Population – Significant Broad Clinical and Quality of Life Improvements for Early Alzheimer's Disease Patients " to be available online as a preprint at medRxiv, and in submission to a peer-reviewed medical journal.
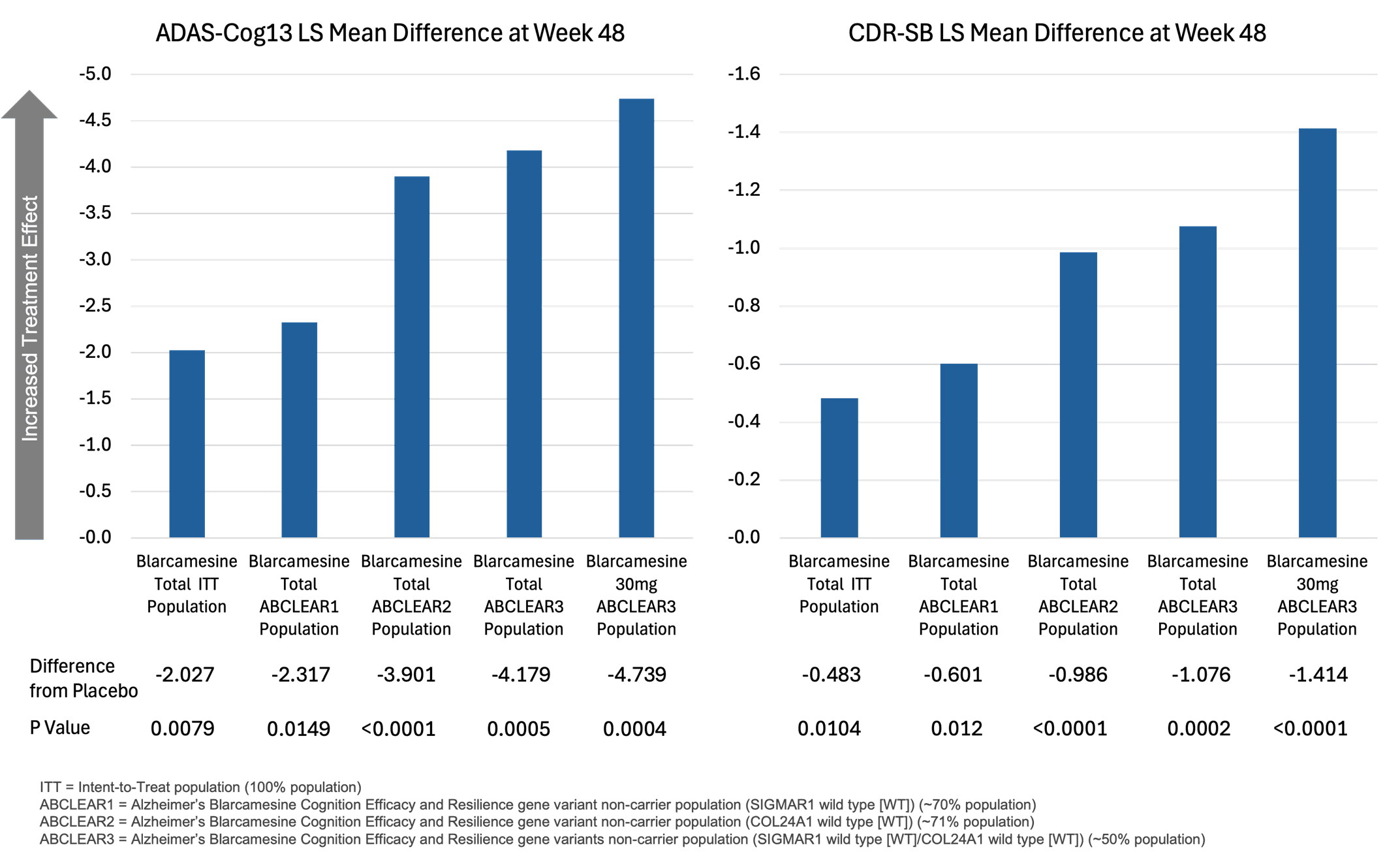
"This publication of blarcamesine, a once-daily oral small molecule, is very exciting, with demonstrated superior clinical efficacy versus approved therapies and slowed neurodegeneration in early Alzheimer's patients," said Marwan Noel Sabbagh, MD, Professor of Neurology, and Chairman of the Anavex Scientific Advisory Board. "Coupled with a strong safety profile and no need for routine MRI monitoring, its unique mechanism of action supports a Precision Medicine approach—potentially benefiting up to 70% of early Alzheimer's disease patients by targeting genetically relevant populations."
"I am very thrilled about the strong cognitive and functional improvements observed in Alzheimer's patients—alongside enhanced Quality of Life and the favorable safety profile," commented Professor of Neurology and Doctor in Neurosciences at the Memory Resources Research Center, the European Neurodegenerative Excellence Center of Montpellier University, France, Audrey Gabelle, MD, PhD and Member of the Anavex Scientific Advisory Board. "Blarcamesine's targeted approach has the potential to significantly reduce both the medical and economic burden of the disease."
"These findings highlight the potential of blarcamesine to reshape Alzheimer's care, particularly within genetically defined populations," said Christopher U. Missling, PhD, President and Chief Executive Officer of Anavex. "Its once-daily oral dosing and favorable safety profile make it a practical and scalable option for early-stage treatment across diverse healthcare settings. We believe this approach could simplify care delivery and expand access for patients navigating the complexities of Alzheimer's disease."
The publication is available at https://www.medrxiv.org/content/10.1101/2025.09.27.25336656v1.full.pdf .
Anavex will continue to evaluate the Phase IIb/III early Alzheimer's disease and ATTENTION-AD trial data, which will be published and presented at international AD conferences.
This release discusses investigational uses of an agent in development and is not intended to convey conclusions about efficacy or safety. There is no guarantee that any investigational uses of such product will successfully complete clinical development or gain health authority approval.
About Anavex Life Sciences Corp.
Anavex Life Sciences Corp. (Nasdaq: AVXL) is a publicly traded biopharmaceutical company dedicated to the development of novel therapeutics for the treatment of neurodegenerative, neurodevelopmental, and neuropsychiatric disorders, including Alzheimer's disease, Parkinson's disease, schizophrenia, Rett syndrome, and other central nervous system (CNS) diseases, pain, and various types of cancer. Anavex's lead drug candidate, ANAVEX ® 2-73 ( blarcamesine ), has successfully completed a Phase 2a and a Phase 2b/3 clinical trial for Alzheimer's disease, a Phase 2 proof-of-concept study in Parkinson's disease dementia, and both a Phase 2 and a Phase 3 study in adult patients and one Phase 2/3 study in pediatric patients with Rett syndrome. ANAVEX ® 2-73 is an orally available drug candidate designed to restore cellular homeostasis by targeting SIGMAR1 and muscarinic receptors. Preclinical studies demonstrated its potential to halt and/or reverse the course of Alzheimer's disease. ANAVEX ® 2-73 also exhibited anticonvulsant, anti-amnesic, neuroprotective, and anti-depressant properties in animal models, indicating its potential to treat additional CNS disorders, including epilepsy. The Michael J. Fox Foundation for Parkinson's Research previously awarded Anavex a research grant, which fully funded a preclinical study to develop ANAVEX ® 2-73 for the treatment of Parkinson's disease. We believe that ANAVEX ® 3-71, which targets SIGMAR1 and M1 muscarinic receptors, is a promising clinical stage drug candidate demonstrating disease-modifying activity against the major hallmarks of Alzheimer's disease in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid, and tau pathologies. In preclinical trials, ANAVEX ® 3-71 has shown beneficial effects on mitochondrial dysfunction and neuroinflammation. Further information is available at www.anavex.com . You can also connect with the Company on Twitter, Facebook , Instagram , and LinkedIn .
Forward-Looking Statements
Statements in this press release that are not strictly historical in nature are forward-looking statements. These statements are only predictions based on current information and expectations and involve a number of risks and uncertainties. Actual events or results may differ materially from those projected in any of such statements due to various factors, including the risks set forth in the Company's most recent Annual Report on Form 10-K filed with the SEC. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement and Anavex Life Sciences Corp. undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof.
For Further Information:
Anavex Life Sciences Corp.
Research & Business Development
Toll-free: 1-844-689-3939
Email: info@anavex.com
Investors:
Andrew J. Barwicki
Investor Relations
Tel: 516-662-9461
Email: andrew@barwicki.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/3796784f-63d7-4b47-9272-3d2f2568ffd7