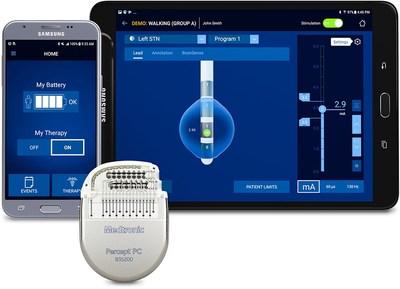
Next-Generation Technology Makes Percept™ the First and Only DBS System with Ability to Capture Patient-Specific Brain Signals for More Personalized, Data-Driven Treatment
Medtronic Canada ULC, a subsidiary of Medtronic plc (NYSE: MDT) – the world's largest medical technology, services, and solutions companies – announced today that it has received a Health Canada licence for the Percept™ PC Deep Brain Stimulation (DBS) system with BrainSense™ Technology. BrainSense technology makes Percept the first and only licensed DBS neurostimulation system with the ability to chronically capture and record brain signals while delivering therapy to patients with neurologic disorders associated with Parkinson's disease, essential tremor, dystonia, or epilepsy. Physicians can now track patient brain signals and correlate these with patient-recorded actions or experiences, such as symptoms, side-effects, or medication intake. This enables more personalized, data-driven neurostimulation treatment. The first implant of the newly licensed device in Canada will occur at Toronto Western Hospital.
"Percept is the first commercially available implantable pulse generator with the ability to record and store the day-to-day brain activity of patients with implanted electrodes for deep brain stimulation," said Dr. Alfonso Fasano , M.D., Ph.D., neurologist and professor of neurology at the University of Toronto . "Neurologists will have, for the first time, the opportunity to monitor their patients' condition well beyond the relatively short time devoted to the doctor-patient interaction in the clinic. Percept will also expand our understanding of brain functioning during physiological and pathological states and set the stage for the development of closed-loop (i.e. adaptive) deep brain stimulation."
DBS is an individualized therapy delivered from a small pacemaker-like device, placed under the skin of the chest or abdomen, to send electrical signals through very thin wires (leads) to a targeted area in the brain related to the symptoms of a neurological disorder, such as Parkinson's disease.
In addition to BrainSense technology, the Percept PC DBS system features several leading-edge innovations, including:
- The only DBS system eligible for 3T and 1.5T full-body MRI scans, providing patients access to cutting-edge medical imaging.
- Smart battery for personalized prediction of remaining battery life providing elevated peace of mind while planning for device replacement.
- Improved battery longevity compared to Medtronic's Activa™ PC neurostimulator (when using similar settings and functionality) in a smaller (reduced volume), ergonomic design for patient comfort.
- Low pulse width (duration of the pulse), providing expanded stimulation options.
- Enhanced Patient Programmer leveraging a user-friendly, custom-configured Samsung mobile device that helps patients manage their therapy more easily.
- Designed to facilitate expanded capabilities in the future via software upgrades – to prepare for what's next in DBS.
"There is nothing currently available that can replace clinical judgement in treating patients. For the first time, this technology gives clinicians feedback directly from the DBS patient's brain," said Mike Daly , vice president and general manager of the Brain Modulation business, which is part of the Restorative Therapies Group at Medtronic. "With such data-driven, patient-specific insights, we believe it can change the standard of care."
For further information on the Percept PC Neurostimulator with BrainSense Technology, please visit: Medtronic.ca/Percept
About Medtronic DBS Therapy
DBS therapy is currently licensed in many locations around the world, including the United States , Europe and Canada , for the treatment Parkinson's disease, essential tremor, dystonia, and epilepsy.
DBS therapy uses a surgically implanted medical device, similar to a cardiac pacemaker, to deliver electrical stimulation to precisely targeted areas of the brain as adjunctive treatment for several neurological disorders. Medtronic was the first in Canada to offer full-body MR Conditional DBS systems for patients to have safe scans anywhere on the body under certain conditions. Since 1987, more than 175,000 Medtronic DBS devices have been implanted worldwide for movement disorders and other indications.
About Medtronic Canada
Proudly serving Canadian healthcare for over 50 years, Medtronic Canada ULC ( www.medtronic.ca ), is a subsidiary of Medtronic plc, the world's largest medical technology, services, and solutions companies – alleviating pain, restoring health, and extending life for millions of people around the world. Serving physicians, hospitals, and patients across the country, Medtronic Canada ULC is headquartered in Brampton, Ontario , with regional offices in Montreal and Vancouver , and a Medtronic Resource Centre in Surrey, BC . The company is focused on collaborating with stakeholders around the world to take healthcare Further, Together.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the U.S. Securities and Exchange Commission. Actual results may differ materially from anticipated results.
SOURCE Medtronic Canada ULC