- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced the first gynecological (GYN) procedures with the Hugo™ robotic-assisted surgery (RAS) system. The six cases included hysterectomies and myomectomies performed by Salomón Zebede, M.D., and Juan Carlos López, M.D., last week at Pacifica Salud Hospital in Panama City, Panama .
"As the cornerstone of our new robotic surgery program, the Hugo RAS system is playing a critical role in bringing the benefits of minimally invasive surgery to more patients in our region," said Dr. Zebede. "It was energizing to perform the very first GYN procedure with the Hugo system, and encouraging to experience firsthand the possibility this technology brings to women's health."
Globally, more than 60% of hysterectomies are performed as open procedures, 1 even though minimally invasive surgery offers fewer complications, shorter hospital stays, and faster return to normal activities. 2-4
Pacifica Salud is the latest institution to join Medtronic's Partners in Possibility Program, a group of pioneering hospitals that will be among the first in the world to use the Hugo RAS system. Earlier this month, the hospital announced its first five urological cases with the Hugo RAS system. Pacifica Salud is also an early adopter of Touch Surgery™ Enterprise, a cloud-based surgical video capture solution that allows surgeons to seamlessly record, analyze, and share surgical video.
"We are witnessing the dawn of a new era in robotic surgery," said Mr. Rafael Cohen , CEO of Pacifica Salud. "That is made possible by the Hugo system, our partnership with Medtronic, and our talented team at Pacifica Salud."
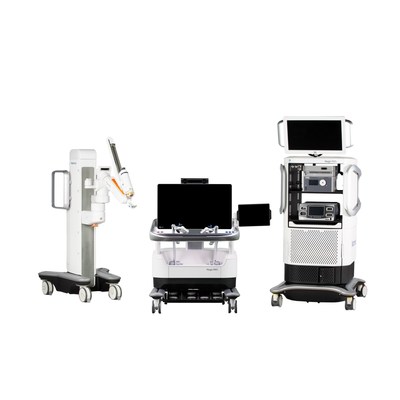
The Hugo RAS system — Medtronic's solution to historic cost and utilization barriers that have kept surgical robotics out of reach for many hospitals — is a modular, multi-quadrant platform designed for a broad range of soft-tissue procedures. In June, Medtronic announced the first clinical procedures with the Hugo system took place in Santiago, Chile . That marked the beginning of the Hugo RAS system patient registry, which is collecting clinical data to support regulatory submissions around the world.
"As an OB/GYN, I'm incredibly passionate about advancing women's health and wellbeing through less invasive solutions that improve outcomes and enable a better quality of life," said Carla Peron , M.D., chief medical officer of the Surgical Robotics business, which is part of Medical Surgical Portfolio at Medtronic. "The first GYN procedures with the Hugo RAS system represent an exciting step toward expanding access to more treatment options, including the benefits of robotic-assisted surgery, to women everywhere."
Medtronic has a longstanding history of making a positive impact on women's health through technology. The Hugo RAS system joins a portfolio of gynecological products designed to enable less invasive surgical treatment of a range of conditions including abnormal uterine bleeding, uterine fibroids, and endometrial cancer.
"I'm proud of the work we're doing at Medtronic with our healthcare partners around the world to improve women's health," said Megan Rosengarten , president of Surgical Robotics. "Advancing access for women to minimally invasive surgical care is a great responsibility and privilege, which makes today's announcement about the Hugo system all the more meaningful."
The Hugo RAS system is not cleared or approved in the U.S. or Europe . Regulatory requirements of individual countries and regions will determine availability and approval or clearance timelines. Touch Surgery Enterprise is available in the U.S. and Europe ; it is not intended to direct surgery, or aid in diagnosis or treatment of a disease or condition.
For more information, visit medtronic.com/hugo .
About Medtronic
Medtronic plc ( www.medtronic.com ), headquartered in Dublin, Ireland , is among the world's largest medical technology, services and solutions companies – alleviating pain, restoring health and extending life for millions of people around the world. Medtronic employs more than 90,000 people worldwide, serving physicians, hospitals and patients in more than 150 countries. The company is focused on collaborating with stakeholders around the world to take healthcare Further, Together.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
- Based on internal estimates and Medtronic report, FY20 market model: procedural volume data.
- Fitch K, Engel T, Bochner A. Cost differences between open and minimally invasive surgery. Managed Care. 2015 Sep;24(9):40–8.
- Tiwari MM, Reynoso JF, High R, Tsang AW, Oleynikov D. Safety, efficacy, and cost effectiveness of common laparoscopic procedures. Surg Endosc. 2011;25(4):1127-1135.
- Roumm AR, Pizzi L, Goldfarb NI, Cohn H. Minimally invasive: minimally reimbursed? An examination of six laparoscopic surgical procedures. Surg Innovation. 2005;12(3):261–287.
| Contacts: | |
| | |
| Gary Jeanfaivre | Ryan Weispfenning |
| Public Relations | Investor Relations |
| +1-203-833-2104 | +1-763-505-4626 |
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/first-gynecological-procedures-performed-with-medtronic-hugo-robotic-assisted-surgery-system-301343980.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/first-gynecological-procedures-performed-with-medtronic-hugo-robotic-assisted-surgery-system-301343980.html
SOURCE Medtronic plc

![]() View original content to download multimedia: https://www.newswire.ca/en/releases/archive/July2021/29/c5290.html
View original content to download multimedia: https://www.newswire.ca/en/releases/archive/July2021/29/c5290.html