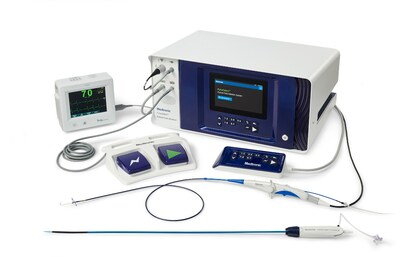
Safe, efficient, and effective treatment for both paroxysmal and persistent atrial fibrillation
Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced that the United States Food and Drug Administration (FDA) has approved the PulseSelect Pulsed Field Ablation (PFA) System for the treatment of both paroxysmal and persistent atrial fibrillation (AF). This is the first PFA technology to receive FDA approval and follows the recent European CE (Conformité Européenne) Mark of the PulseSelect PFA system in November.
"Launching the first FDA-approved PFA technology is not just a milestone; the PulseSelect PFA system is setting a new standard in safety for AF ablation with excellent efficacy and efficiency 1 . It's a major step towards fulfilling our vision of providing disruptive electrophysiology solutions for patients," said Rebecca Seidel , SVP and President of the Cardiac Ablation Solutions business, which is part of the Cardiovascular Portfolio. "The PulseSelect PFA system, together with the CE Marked Affera™ mapping and ablation system and our strong Cryo platform, enables us to provide a broad portfolio of solutions to clinicians and their patients, all developed with years of research and supported by compelling scientific evidence."
The PulseSelect PFA system was engineered with differentiated safety features and provides rapid, effective pulmonary vein isolation (PVI) through consistent and predictable energy delivery and catheter maneuverability. The system is designed to enable a seamless transition to PFA in a clinician's preferred workflow 2 . The PulseSelect PFA system's safety, efficacy, and efficiency is supported by the PULSED AF study, which showed a 0.7% safety event rate and clinical success rates of 80% in both paroxysmal and persistent AF patients 1 .
"The PulseSelect PFA system ushers the EP community to a new era of safe, effective, and efficient AF ablation that overcomes many challenges in our current practice," said Dr. Amin Al-Ahmad , clinical cardiac electrophysiologist at St. David's Medical Center in Austin, TX and one of 67 global operators in the PULSED AF trial. "In my clinical experience with the catheter, it was designed for AF ablation procedures. The learning curve in using the catheter and system is short, and the catheter enables the operator to deliver fast and controlled pulsed field energy for AF ablation."
The PulseSelect PFA system also includes the following:
- Designed as a plug-and-play system, PulseSelect can be used with any mapping system or with just fluoroscopy 2 .
- Built-in safety features such as a phrenic nerve test pulse, a non-therapeutic low voltage pulse that provides a preemptive assessment of catheter proximity to the phrenic nerve prior to delivering a therapeutic application.
- Fixed spacing for the nine-electrode catheter, which produces a predictable and consistent electric field for contiguous ablation 2 . In addition to ablation, the nine electrodes can also be used for pacing and sensing.
- The small, 9Fr bidirectional catheter enhances maneuverability and access to various anatomical structures and is compatible with a 10Fr sheath, including the custom bidirectional FlexCath Contour™ sheath.
"We are thrilled to see the continuous innovation of our legacy Cryoablation portfolio alongside the approval of the PulseSelect PFA system in the U.S.," said Khaldoun Tarakji , MD MPH, Chief Medical Officer of the Cardiac Ablation Solutions business at Medtronic. "Every patient deserves the best care. What motivates all of us at Medtronic is the privilege of serving patients by empowering electrophysiologists globally with the safest and most effective ablation technologies that seamlessly integrate with their workflows and enable them to tailor therapy based on their patients' needs."
The PulseSelect PFA system is also the first FDA Breakthrough-designated PFA technology to be approved. The designation is intended to help patients gain more timely access to medical devices that have the potential to make a significant impact in the diagnosis or treatment of life-threatening conditions.
Commercialization of the PulseSelect PFA system will start in early 2024.
About Atrial Fibrillation and Pulsed Field Ablation
AF is one of the most common and undertreated heart rhythm disorders, affecting more than 60 million people worldwide 3 . AF is a progressive disease, meaning it can become worse over time and can increase the risk of serious complications including heart failure, stroke and increased risk of death. 4-7 Current ablation technologies rely on thermal effects to target cardiac tissue and risk damage to additional collateral structures in the heart. PFA is a breakthrough ablation technology that uses pulsed electric fields to efficiently isolate the pulmonary veins for the treatment of AF. Because the mechanism of cell death is non-thermal, the risk of collateral structure damage is potentially lower.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland , is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com , and follow @Medtronic on LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
- Verma A, et al. Circulation. 2023;147:1422-1432
- Medtronic data on file. November 2023 .
- Roth GA, Mensah GA, Johnson CO et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982-3021.
- Miyasaka Y, Barnes ME, Bailey KR, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol 2007;49:986-92.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020.
- Wolf PA , Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8.
- Lubitz SA, Moser C, Sullivan L, et al. Atrial fibrillation patterns and risks of subsequent stroke, heart failure, or death in the community. J Am Heart Assoc 2013;2:e000126
| Contacts: | | |
| | | |
| Allison Kyriagis | Ryan Weispfenning | |
| Public Relations | Investor Relations | |
| +612-750-6061 | +1-763-505-4626 | |
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/medtronic-creates-history-with-fda-approval-of-its-novel-pulseselect-pulsed-field-ablation-system-to-treat-atrial-fibrillation-302014766.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/medtronic-creates-history-with-fda-approval-of-its-novel-pulseselect-pulsed-field-ablation-system-to-treat-atrial-fibrillation-302014766.html
SOURCE Medtronic plc