
October 08, 2023
Ovarian cancer diagnostics company, Cleo Diagnostics Limited (ASX:COV) (CLEO, or the Company) confirms the delivery of early milestones in its development program targeting the initial surgical triage test market for its simple and accurate blood test.
Highlights
- Selection of biomarkers panel for Cleo’s ovarian cancer test-kit has been finalised
- Antibody development has advanced increasing confidence for commercial assay development and upscaling for commercial manufacturing
- The Company is in final stages evaluating four commercial antibody manufacturing partners
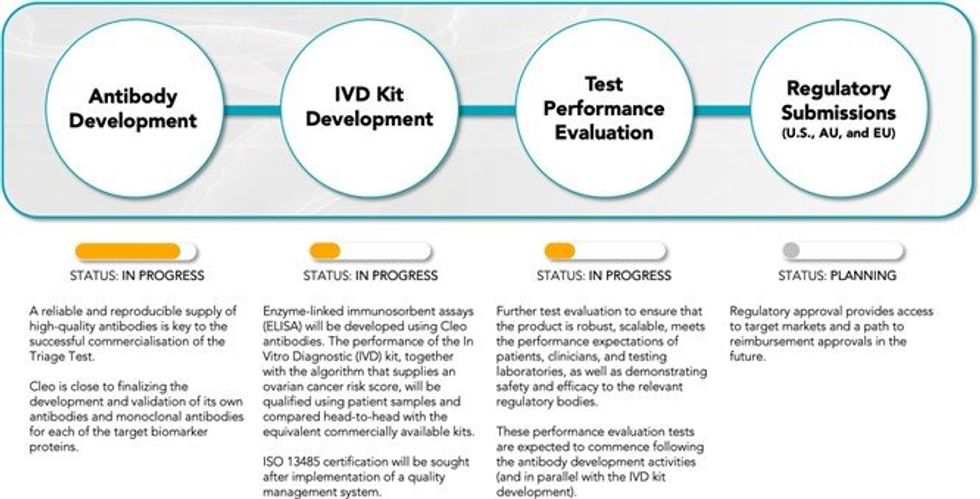
TEST-KIT BIOMARKERS PANEL FINALISED
Cleo has finalised the selection of biomarkers to be used in its ovarian cancer test-kit, along with completing the development for a prototype of the proprietary scoring algorithm. The performance metrics of the test were evaluated in a clinical study of 334 patients, the results of which are being prepared for publication in a peer-reviewed medical journal. The Company expects the publication outcome to be reported to the market by the end of CY2023. The data cannot be released prior to publication due to the nature of the peer-review process. Concurrently, Cleo is also preparing a further patent application based on the findings.
ANTIBODY DEVELOPMENT
A key objective for the Company is to develop its own antibodies and target proteins which will allow control of supply, quality, cost and high-performance of key reagents that will underpin the consistent and reliable manufacture of test-kits. Cleo can confirm that Surface Plasmon Resonance Analysis has shown that the core antibodies of the CXCL10 active ratio test are binding to their respective targets with high affinity and are suitable for commercial assay development and upscaling in commercial manufacturing. Hybridomas to produce the supporting biomarker antibodies are also well progressed, with expected completion of the full test-kit panel in Q2 CY2024.
Commenting on the early development progress, CLEO Chief Executive, Richard Allman, said:
“The commercial foundation for our ovarian cancer test-kit targeting the initial surgical triage market is coming together quickly. We are running a number of initiatives in parallel which are designed to place the Company in a strong position to achieve key milestones set this financial year.”
SELECTION PROCESS FOR ANTIBODY MANUFACTURING PARTNER
Cleo is in the late stages of evaluating four commercial antibody manufacturing partners as part of a robust tender process. The evaluation process considers a competitive review of the capabilities of each potential partner to ensure that the partner ultimately selected can deliver commercial product to the standard required by Cleo, which is largely set by regulatory bodies such as the Food and Drug Administration (FDA) and potential customer groups.
Click here for the full ASX Release
This article includes content from CLEO Diagnostics, licensed for the purpose of publishing on Investing News Australia. This article does not constitute financial product advice. It is your responsibility to perform proper due diligence before acting upon any information provided here. Please refer to our full disclaimer here.
COV:AU
The Conversation (0)
21 May 2024
Cleo Diagnostics
Revolutionising Ovarian Cancer Diagnosis Through Accurate and Early Detection
Revolutionising Ovarian Cancer Diagnosis Through Accurate and Early Detection Keep Reading...
17 March 2025
CLEO Delivers Commercial Development Milestone
Cleo Diagnostics (COV:AU) has announced CLEO Delivers Commercial Development MilestoneDownload the PDF here. Keep Reading...
27 February 2025
Appendix 4D and Half Yearly Accounts
Cleo Diagnostics (COV:AU) has announced Appendix 4D and Half Yearly AccountsDownload the PDF here. Keep Reading...
31 January 2025
Quarterly activities and cashflow report
Cleo Diagnostics (COV:AU) has announced Quarterly activities and cashflow reportDownload the PDF here. Keep Reading...
09 December 2024
CLEO Further Expands Ovarian Cancer Trial with Siles Health
Cleo Diagnostics (COV:AU) has announced CLEO Further Expands Ovarian Cancer Trial with Siles HealthDownload the PDF here. Keep Reading...
26 November 2024
The Royal Women's Hospital Joins CLEO Ovarian Cancer Trial
Cleo Diagnostics (COV:AU) has announced The Royal Women's Hospital Joins CLEO Ovarian Cancer TrialDownload the PDF here. Keep Reading...
28 January
Seegnal Presents Real-World Evidence on Reducing Fall Risk in Geriatric Patients at Caltcm Summit
Seegnal Inc. (TSXV: SEGN) ("Seegnal" or the "Corporation"), a global leader in AI-enhanced prescription intelligence, today announced real-world clinical results demonstrating how medication governance may reduce fall-risk drivers in older adults -- a significant clinical and financial challenge... Keep Reading...
05 January
Pathways to Commercialising Biotech Innovations
In the medical technology industry, innovation is only the first step. While key to long-term success, innovation is only as good as a company’s commercialisation strategy. Once a technology has been developed and proven, the organisation must then embark on a process to commercialise it for... Keep Reading...
25 February 2025
HeraMED Signs Strategic Collaboration Agreement with Garmin Health
HeraMED Limited (ASX: HMD), a medical data and technology company leading the digital transformation of maternity care, is delighted to announce it has entered into a collaboration agreement with Garmin (NYSE: GRMN), a leading global provider of smartwatches and GPS-enabled products, aimed at... Keep Reading...
23 January 2025
Cyclopharm Signs US Agreement with HCA Healthcare for Technegas®
Cyclopharm Limited (ASX: CYC) is pleased to announce the signing of a major contract with Hospital Corporation of America Healthcare (HCA), one of the largest single healthcare providers in the United States. This agreement marks a significant milestone for the company which will allow the... Keep Reading...
23 January 2025
CONNEQT App Launches in USA as Pulse Deliveries Commence
Cardiex Limited (CDX:AU) has announced CONNEQT App Launches in USA as Pulse Deliveries CommenceDownload the PDF here. Keep Reading...
16 January 2025
Revolutionizing Women's Health: Antifungal Innovation Brings New Investment Opportunities
The intersection of women's health and antifungal innovation represents a pivotal moment in healthcare, offering both transformative medical advancements and compelling investment opportunities. The groundbreaking developments in antifungal treatments specifically targeting women's health issues... Keep Reading...
Latest News
Interactive Chart
Latest Press Releases
Related News
TOP STOCKS
American Battery4.030.24
Aion Therapeutic0.10-0.01
Cybin Corp2.140.00









