April 29, 2022
BriaCell Therapeutics (NASDAQ:BCTX, BCTXW;TSX:BCT) is a clinical phase biotechnology company developing innovative immunotherapies for treating cancer.
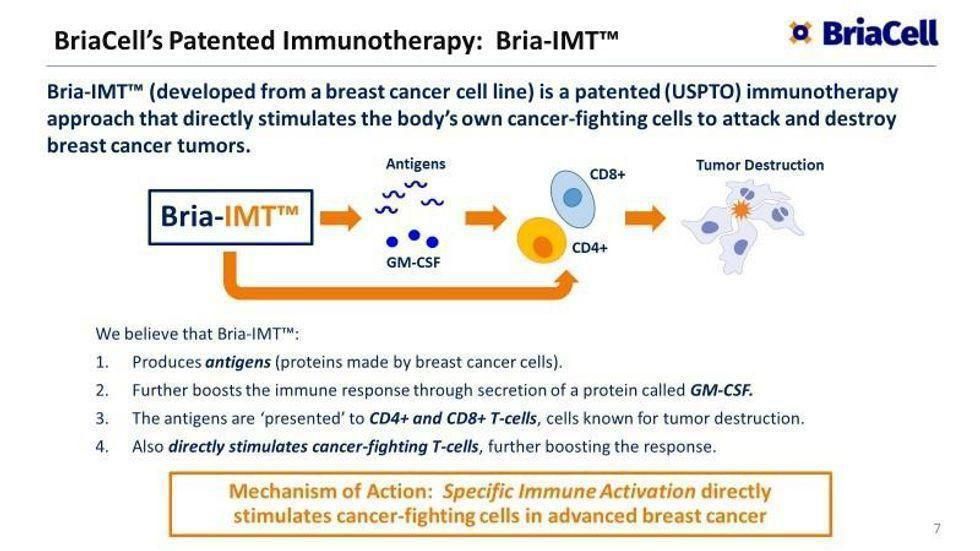
With a recently-awarded Fast Track status from the FDA, BriaCell's lead candidate, Bria-IMT™, is an immunotherapy currently being developed to treat advanced breast cancer, which is the expected cause of over 43,000 deaths in the US in 2022. Bria-IMT™achieved positive proof of concept, showing outstanding safety and efficacy data. Importantly, preliminary efficacy data was similar, or superior to, those of other approved breast cancer drugs when they were at a similar stage of clinical development. After significant success, BriaCell received FDA Fast Track designation for this targeted immunotherapy approach.
Company Highlights
- April 2022: BriaCell receives FDA fast-track approval for targeted breast cancer immunotherapy.
- February 2022: BriaCell appoints renowned oncologist, Dr. Giuseppe Del Priore as chief medical officer, appoints immunologist Dr. Alexander Kharazi to its advisory board, and adds two clinical trial sites to accelerate patient enrollment.
- December 2021: BriaCell announces insiders' intention to purchase up to 10 percent of public market securities.
- September 2021: BriaCell announces securities buyback to purchase up to 10 percent of common shares and up to 10 percent of listed warrants.
- July 2021: BriaCell Phase I/IIa clinical trial combination study in advanced breast cancer patients opens for enrollment.
- June 2021: BriaCell Therapeutics announces closing of US$27.2 million private placement
- June 2021: BriaCell Reports 12.0 months overall survival benefit in advanced breast cancer; 100 percent resolution of ‘eye-bulging’ tumor
- February 2021: BriaCell announces closing of US$25 million public offering and listing on NASDAQ.
This BriaCell Therapeutics company profile is part of a paid investor education campaitn.*
BCT:CA
The Conversation (0)
10 November 2022
BriaCell
Developing Personalized Off-The-Shelf Immunotherapy Treatments for Cancer Patients
Developing Personalized Off-The-Shelf Immunotherapy Treatments for Cancer Patients Keep Reading...
Latest News
Interactive Chart
Latest Press Releases
Related News
TOP STOCKS
American Battery4.030.24
Aion Therapeutic0.10-0.01
Cybin Corp2.140.00