- First-of-its-kind report is available at www.naturallyyouwithHA.com
- Deep dive into category education and data on HA injectable fillers and the natural-looking results they can achieve
- Allergan Aesthetics, an AbbVie company, has launched the " Naturally You with Injectable Hyaluronic Acid Fillers" campaign aimed to providing clear, factual education about HA injectable fillers. This initiative focuses on correcting misinformation to elevate understanding and celebrate the safe, natural-looking outcomes possible with hyaluronic acid injectable fillers.
"Our goal with Naturally You with Injectable Hyaluronic Acid Fillers campaign is to set the record straight on hyaluronic acid injectable fillers for our healthcare providers, our patients and the category," said Glen Curran , Senior Vice President, U.S. Aesthetics, Allergan Aesthetics. "By providing facts, real-world satisfaction data and expert insights, we hope to truly show how HA fillers are a safe and effective way to address aesthetic concerns."
The new report, The Hyaluronic Acid Injectable Fillers Report, is at the center of the campaign and sheds light on the current use, opinions and personal experiences around of HA injectable fillers.
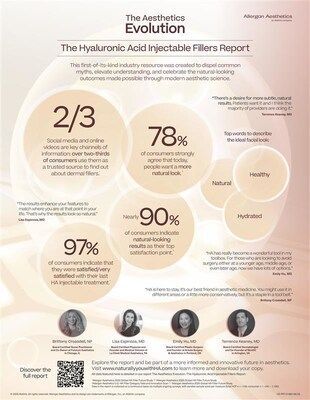
Key stats include:
- Nearly 90% of consumers indicate they are satisfied with the outcome, noting natural-looking results as their top satisfaction point. 1
- 78% of consumers strongly agree that today, people want a more natural look.
- Over two-thirds of consumers use social media and online videos to learn about HA fillers.
- Over 75% of consumers believe that more people will use injectable HA fillers in the future. 2
The report draws from extensive research, including analysis of consumer and business media, cultural conversations, social trends, and proprietary U.S. data from Allergan Aesthetics, the market-leader in HA injectable fillers. Insights were further validated through interviews with four leading aesthetics experts from key U.S. markets: Brittony Croasdell , NP, Chicago, IL ; Lisa Espinoza , MD, PA; Emily Hu , MD, Portland, OR ; and Terrence Keaney , MD, Washington, DC —and a foreword by award-winning beauty journalist, Jamie Rosen , reflecting on how far the aesthetics category has come and what's ahead.
The Hyaluronic Acid Injectable Fillers Report is the first in the Aesthetics Evolution Report Series, which will continue to provide insights into the aesthetics industry.
As part of the Naturally You with Injectable Hyaluronic Acid Fille rs campaign, Allergan Aesthetics will partner with healthcare providers and consumer influencers, to support education on the category and promotion of healthy, natural-looking outcomes consumer desire today.
Join Allergan Aesthetics in reshaping the narrative around HA injectable fillers. Explore the report and be part of a more informed future in aesthetics.
Visit www.naturallyyouwithHA.com to learn more and download your copy of The Hyaluronic Acid Injectable Fillers Report .
About Allergan Aesthetics
At Allergan Aesthetics, an AbbVie company, we develop, manufacture, and market a portfolio of leading aesthetics brands and products. Our aesthetics portfolio includes facial injectables, body contouring, plastics, skin care, and more. Our goal is to consistently provide our customers with innovation, education, exceptional service, and a commitment to excellence, all with a personal touch. For more information, visit www.AllerganAesthetics.com .
About AbbVie
AbbVie's mission is to discover and deliver innovative medicines and solutions that solve serious health issues today and address the medical challenges of tomorrow. We strive to have a remarkable impact on people's lives across several key therapeutic areas – immunology, oncology, neuroscience, and eye care – and products and services in our Allergan Aesthetics portfolio. For more information about AbbVie, please visit us at www.abbvie.com . Follow @AbbVie on LinkedIn, Facebook , Instagram , X (formerly Twitter) , and YouTube.
Forward-Looking Statements
Some statements in this news release are, or may be considered, forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995. The words "believe," "expect," "anticipate," "project" and similar expressions and uses of future or conditional verbs, generally identify forward-looking statements. AbbVie cautions that these forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those expressed or implied in the forward-looking statements. Such risks and uncertainties include, but are not limited to, challenges to intellectual property, competition from other products, difficulties inherent in the research and development process, adverse litigation or government action, and changes to laws and regulations applicable to our industry. Additional information about the economic, competitive, governmental, technological and other factors that may affect AbbVie's operations is set forth in Item 1A, "Risk Factors," of AbbVie's 2023 Annual Report on Form 10-K, which has been filed with the Securities and Exchange Commission, as updated by its subsequent Quarterly Reports on Form 10-Q. AbbVie undertakes no obligation, and specifically declines, to release publicly any revisions to forward-looking statements as a result of subsequent events or developments, except as required by law.
U.S. Media:
Kate McShane
Corporate Communications
Kate.mcshane@allergan.com
References:
- Allergan Aesthetics U.S. HA Filler Category Data and Innovation Scan
- Allergan Aesthetics 2025 Global HA Filler Future Study
Data in the report is collected on a continuous basis via multiple ongoing surveys, with variable sample sizes per measure (total HCP n = ~100; consumer n = ~241 – 1,180).
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/allergan-aesthetics-launches-campaign-to-educate-consumers-about-hyaluronic-acid-ha-injectable-fillers-302544294.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/allergan-aesthetics-launches-campaign-to-educate-consumers-about-hyaluronic-acid-ha-injectable-fillers-302544294.html
SOURCE AbbVie