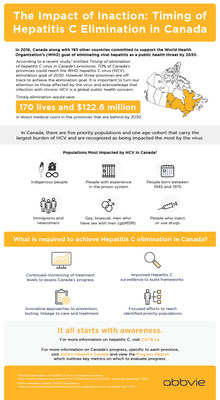
- Timing of elimination of the hepatitis C virus (HCV) in Canada's provinces indicates 70% of provinces could reach the World Health Organization's (WHO) HCV elimination target of 2030, however three of Canada's provinces — two of them the most populous in the country — are off track to achieve this hepatitis C elimination goal. 1
- Timely elimination would save 170 lives and $122.6 million in direct medical costs in these three provinces by 2030. 1
- The Progress Report developed by Action Hepatitis Canada outlines key metrics on which to evaluate HCV elimination progress in Canada , specific to each province. 2
- In Canada , there are five priority populations and one age-cohort that carry the largest burden of HCV and are recognized as being affected the most by the virus.
ABBVie (NYSE: ABBV) supports a wide range of efforts to help elevate and prioritize hepatitis C virus (HCV) elimination. With a recent publication indicating 70% of Canada's provinces are on track to reach HCV elimination by the World Health Organization's (WHO) initial proposed target of 2030 1 it is important to turn our attention on those affected by the virus and acknowledge that infection with chronic HCV is a global public health concern.
In 2016, Canada was one of the 194 countries that committed to support the World Health Organization's (WHO) goal of eliminating viral hepatitis as a public health threat by 2030. With the remarkable progress in HCV therapy, offering the ability to cure patients, this goal seemed possible.
"With the decline in treatment across Canada , it is particularly critical we continue to monitor treatment levels to assess Canada's progress to HCV elimination," said Jordan J Feld, MD MPH, Interim Director, Toronto Centre for Liver Disease, University Health Network, University of Toronto . "We need to continue to pursue novel approaches to case finding and linkage to care, as well as work closely with identified priority populations to ensure that they are able to seek prevention and treatment services without facing stigma and other barriers in the health care system. At the policy level, we need to improve our data sharing abilities across the country to ensure we can track our progress toward elimination."
Populations Most Affected by HCV in Canada 3
- Indigenous people
- People with experience in the prison system
- People born between 1945 and 1975
- Immigrants and newcomers
- Gay, bisexual, men who have sex with men (gbMSM)
- People who inject or use drugs
" Canada has made great strides toward the elimination of hepatitis C. However, there is a lot more work to be done, and the tactics that got us to this point will not necessarily get us to elimination," said Jennifer van Gennip , Executive Director, Action Hepatitis Canada. "Our mandate is to hold the federal and provincial governments accountable to provide the policies and resourced plans to achieve our goal, with focused efforts on priority populations within Canada ."
A look at national treatment data from January 2019 to November 2020 4 confirmed the decreasing trend in treatment levels nationally - a year-over-year decline of 31% in total treatment levels between 2019 and 2020. The report notes that this drop could be due to the disruptions to the healthcare system caused by the COVID-19 pandemic but could also reflect the saturation of treatment among those already linked to care and the difficulties with finding and engaging with individuals and populations not well served by our various healthcare systems. 1
"Everyone has a part to play in eliminating viral hepatitis as it will take more than medicine to achieve this goal," said Tracey Ramsay , Vice-president and General Manager, AbbVie Canada. "AbbVie is committed to partnering with stakeholders to implement sustainable solutions that allow more patients to be screened, linked to care, and treated in a timely manner, especially for those vulnerable patient populations that have lost access to our healthcare system as a result of the pandemic."
Given the available evidence, Canada's momentum towards timely HCV elimination may be jeopardized if diagnosis and treatment are not maintained at appropriate levels. Improved HCV surveillance to build frameworks and innovative approaches to prevention, testing, linkage to care and treatment to achieve this goal is required.
About Hepatitis C
An estimated 250,000 people in Canada are living with chronic hepatitis C but as many as 44% are not aware that they have the disease. 5 Left undiagnosed and untreated, chronic hepatitis C can lead to cirrhosis, liver cancer or liver failure. Currently, hepatitis C is the leading indication for liver transplant in Canada . 6 AbbVie supports a range of efforts to help elevate and prioritize HCV elimination because we know achieving the shared goal of elimination by 2030 will take more than medicine. It will take transparent and collaborative partnerships with all stakeholders – industry, healthcare providers, healthcare systems, patient groups and their support networks. Joint efforts and maximizing the time we have left will enable us to reach this goal.
About AbbVie
AbbVie's mission is to discover and deliver innovative medicines that solve serious health issues today and address the medical challenges of tomorrow. We strive to have a remarkable impact on people's lives across several key therapeutic areas: immunology, oncology, neuroscience, eye care, virology, women's health and gastroenterology, in addition to products and services across its Allergan Aesthetics portfolio. For more information about AbbVie, please visit us at www.abbvie.ca . Follow AbbVie Canada on Twitter , Instagram and LinkedIn.
| | 1 Timing of elimination of hepatitis C virus in Canada's provinces. https://canlivj.utpjournals.press/doi/full/10.3138/canlivj-2022-0003 . Accessed November 2022. |
| | 2 Action Hepatitis Canada. Progress Toward Viral Hepatitis Elimination in Canada. 2021 Report. https://www.actionhepatitiscanada.ca/uploads/8/3/3/9/83398604/ahc_progress_report_2021.pdf . Accessed November 2022. |
| | 3 Action Hepatitis Canada. Priority Populations. https://www.actionhepatitiscanada.ca/priority-populations.html . Accessed November 2022. |
| | 4 IQVIA GPM National Audit for HCV/Direct Acting Antivirals Market, January 2019–November 2020. |
| | 5 Canadian Liver Foundation. https://www.liver.ca/hepatitis-c-warning/ . Accessed November 2022. |
| | 6 Canadian Liver Foundation. https://www.liver.ca/how-you-help/advocate/ . Accessed November 2022. |
SOURCE AbbVie Canada

![]() View original content to download multimedia: https://www.newswire.ca/en/releases/archive/November2022/24/c4530.html
View original content to download multimedia: https://www.newswire.ca/en/releases/archive/November2022/24/c4530.html