The company's newest Simplera™ CGM is 50% smaller than its previous generation with a simple insertion and improved user experience
Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced CE (Conformité Européenne) Mark approval for its new all-in-one, disposable Simplera™ continuous glucose monitor (CGM) featuring a simple, two-step insertion process. The company's newest no-fingerstick sensor does not require over tape and is seamlessly integrated with the InPen™ smart insulin pen, which provides real-time, personalized dosing guidance to help simplify diabetes management. Medtronic will begin a phased launch at the European Association for the Study of Diabetes (EASD) 59th Annual Meeting in Hamburg, Germany on Oct. 2-6, 2023 .
"Patients with diabetes can get overwhelmed by the sheer number of decisions they need to make on a daily basis. As a physician, I appreciate the ability to introduce this solution by Medtronic as it provides real-time, personalized guidance to help patients stay in range. For instance, when it detects someone is consuming a meal and their glucose levels are trending high, it alerts the person to help make diabetes management easier and provides peace of mind," said Dr. Sandra Schlüter, Endocrinologist, Germany , Head of AGDF.
"Despite the rapid adoption of CGM over the past decade, less than 30% of individuals on MDI therapy using a CGM achieve glycemic targets — highlighting a significant unmet need. 1-4 We're excited to help more people to reach their goals with our advanced algorithm in InPen™ powered by our smallest and most comfortable CGM to-date," said Que Dallara, EVP and President, Medtronic Diabetes. "This newest addition of a Smart MDI solution to our holistic portfolio demonstrates our commitment to meeting people where they are in their diabetes journey with simplified solutions that help make life with diabetes easier."
Simplera™ is indicated for ages 2+ and compatible with iOS and Android. Simplera™ is not approved by the FDA and is limited to investigational use in the U.S. Medtronic's automated insulin delivery (AID) system integrated with this next-generation sensor is currently under review for CE Mark and is not commercially available in the U.S. or in Europe.
About Smart MDI
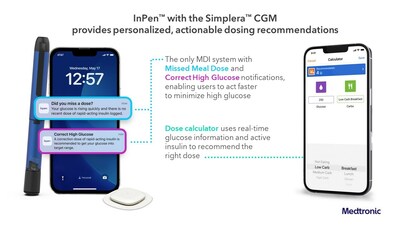
The Medtronic Smart MDI system is the first to seamlessly integrate real-time CGM with a smart insulin pen powered by an adjustable algorithm that delivers personalized dosing recommendations. The InPen TM combined with the new Simplera TM CGM provides users with actionable insights that reduce guesswork and complicated manual calculations to help simplify diabetes management.
About the Diabetes Business at Medtronic ( www.medtronicdiabetes.com )
Medtronic Diabetes is on a mission to alleviate the burden of diabetes by empowering individuals to live life on their terms, with the most advanced diabetes technology and always-on support when and how they need it. We've pioneered first-of-its-kind innovations for over 40 years and are committed to designing the future of diabetes management through next-generation sensors (CGM), intelligent dosing systems, and the power of data science and AI while always putting the customer experience at the forefront.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland , is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across more than 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn .
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
1 Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, Maahs DM, Tamborlane WV, Bergenstal R, Smith E, Olson BA, Garg SK. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019 Feb;21(2):66-72. doi: 10.1089/dia.2018.0384. Epub 2019 Jan 18. Erratum in: Diabetes Technol Ther. 2019 Apr;21(4):230. PMID: 30657336; PMCID: PMC7061293.
2 Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of Real-time Continuous Glucose Monitoring With Glycemic Control and Acute Metabolic Events Among Patients With Insulin-Treated Diabetes. JAMA. 2021;325(22):2273–2284. doi:10.1001/jama.2021.6530.
3 Nathanson D, Svensson AM, Miftaraj M, Franzén S, Bolinder J, Eeg-Olofsson K. Effect of flash glucose monitoring in adults with type 1 diabetes: a nationwide, longitudinal observational study of 14,372 flash users compared with 7691 glucose sensor naive controls. Diabetologia. 2021 Jul;64(7):1595-1603. doi: 10.1007/s00125-021-05437-z. Epub 2021 Mar 27. PMID: 33774713; PMCID: PMC8187189.
4 Leelarathna L, Evans ML, Neupane S, Rayman G, Lumley S, Cranston I, Narendran P, Barnard-Kelly K, Sutton CJ, Elliott RA, Taxiarchi VP, Gkountouras G, Burns M, Mubita W, Kanumilli N, Camm M, Thabit H, Wilmot EG; FLASH-UK Trial Study Group. Intermittently Scanned Continuous Glucose Monitoring for Type 1 Diabetes. N Engl J Med. 2022 Oct 20;387(16):1477-1487. doi: 10.1056/NEJMoa2205650. Epub 2022 Oct 5 . PMID: 36198143.
| Contacts: | |
| | |
| Ashley Patterson | Ryan Weispfenning |
| Public Relations | Investor Relations |
| +1-818-576-3025 | +1-763-505-4626 |
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/medtronic-diabetes-announces-ce-mark-for-new-simplera-cgm-with-disposable-all-in-one-design-301934170.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/medtronic-diabetes-announces-ce-mark-for-new-simplera-cgm-with-disposable-all-in-one-design-301934170.html
SOURCE Medtronic plc