- NORTH AMERICA EDITIONAustraliaNorth AmericaWorld
Overview
Ocumetics Technology Corp. (TSXV:OTC, OTCQB:OTCFF, FRA:2QBO) is at the forefront of research and product development, focusing on a groundbreaking vision enhancement product - the Ocumetics Accommodating Lens.
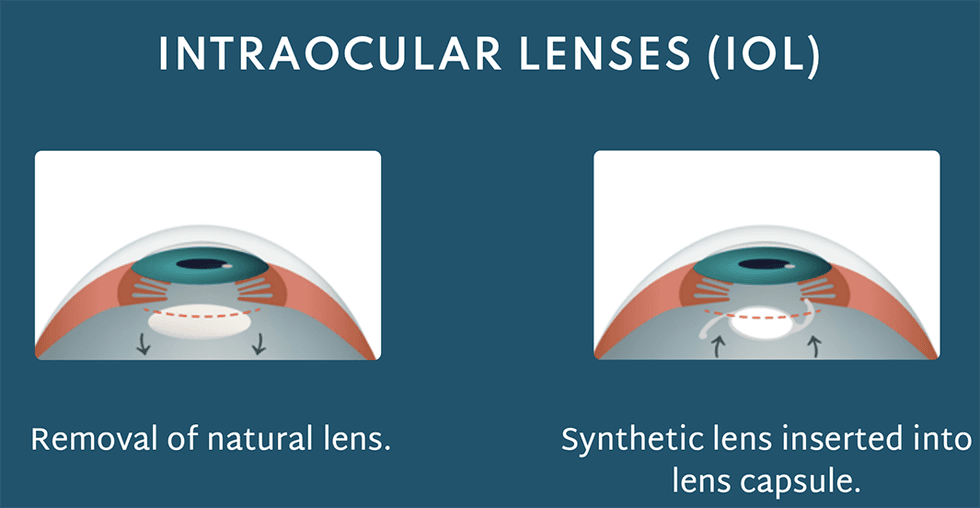
This intraocular lens (IOL) is engineered to seamlessly shift the eye's focus from close to far distances without any noticeable lag. This process is called accommodation.
To grasp the innovation of Ocumetics’ products, it's essential to understand the workings of the eye. The cornea, a transparent front layer, bends incoming light rays to form images on the retina - the thin, light-sensitive, nerve-rich tissue at the back of the eyes. Working in concert, the pupil and iris regulate light entry, aiding vision adjustment for various distances.
As individuals age or suffer injuries, the eye's natural lens loses its flexibility to bend light rays accurately, leading to deteriorating eyesight and potential conditions like cataracts with or without astigmatism.
Addressing this issue, Ocumetics has designed a very unique IOL that is surgically implanted to restore a patient’s vision. This lens is designed to harmoniously align with the eye's natural mechanisms, enhancing vision by appropriately bending light rays. Notably, it's designed to last a lifetime, presenting a significant potential advancement over other cataract technologies.
The journey to introduce this transformative product to the public is well underway. Ocumetics is positioned to complete a pivotal biocompatibility study in October 2023, followed by a planned first in-human studies in Q1 2024.
Human clinical trials for proof-of-concept are expected to commence by December 2024, in collaboration with esteemed institutions in the United States, Canada and Europe. The company aims for US FDA approval within three-and-a-half years, potentially revolutionizing the vision care market. A dedicated team of highly experienced eye care experts, scientists and business leaders propels Ocumetics forward, ushering this transformative product into the market.
Company Highlights
- Ocumetics Technology Corp. is an R&D company developing a vision enhancement product with the potential to disrupt the vision correction industry.
- The Ocumetics Accommodating Lens is an intraocular lens designed to allow the human eye to shift between close and long-distance images without perceptible adjustment lag.
- Ocumetics’ product is surgically implanted and is designed to enhance the natural processes, allowing the patient’s eyes to bend light properly for better vision.
- The Ocumetics Accommodating Lens has substantially completed a series of design improvements, animal studies, surgical procedure development and biocompatibility studies and will soon move toward human clinical trials, slated to begin in Q1 2024.
- The company aims to receive full US FDA approval within three-and-a-half years and bring its product to a projected intraocular lens market segment of US$6.4 billion by 2030 (Delveinsight 2022).
- An expert team with experience in clinical eye care, R&D and corporate development is leading the company toward bringing its transformative product to market.
Get access to more exclusive Medical Device Investing Stock profiles here


