NurExone Biologic Inc. (TSXV: NRX,OTC:NRXBF) (OTCQB: NRXBF) (FSE: J90) (" NurExone " or the " Company "), a preclinical-biotechnology company developing exosome-based therapies for central nervous system injuries, today announced meaningful progress in its long-term clinical-readiness strategy. The Company has begun the process of evaluating potential Israeli production partners to support small-scale GMP-based manufacturing of its lead candidate, ExoPTEN, in order to test the drug in under a First-in-Human Use program, pending regulatory approval.
Dr. Lior Shaltiel, Chief Executive Officer, noted: "ExoPTEN brings together a powerful synergistic impact with highly active exosomes produced, combined with a targeted siRNA. As we advance our manufacturing plans, small clinical grade batches will position us to prepare a First-in-Human use submission in the future, if and when the regulatory pathway supports it." He added, "ExoPTEN is being developed for both acute spinal cord injury and optic nerve injury, although it has not yet been determined which indication will be prioritized for a first-in-human program."
In parallel, NurExone reported new scientific data showing strong biological activity in its exosomes compared with commercially available exosomes, reinforcing confidence in the platform as the Company advances toward human studies.
Early Manufacturing in Israel: A Key Step Toward U.S. Scale-Up
NurExone is currently evaluating several Israeli manufacturing organizations with expertise in advanced biologics and Good Manufacturing Practices (" GMP ")-aligned systems for small-scale ExoPTEN manufacturing runs aligned with future clinical requirements.
"This is a strategically important next phase of operational de-risking," said Dr. Ina Sarel, Head of CMC, Quality and Regulatory Affairs. "Israel is the starting point for our small scale GMP-production of exosomes and process validation, after which exosome production will transition to the United States for scale-up."
NurExone emphasized that no compassionate-use or clinical manufacturing is being initiated at this stage. All such activities would be subject to regulatory engagement and approval.
New Scientific Data Reinforce Platform Strength
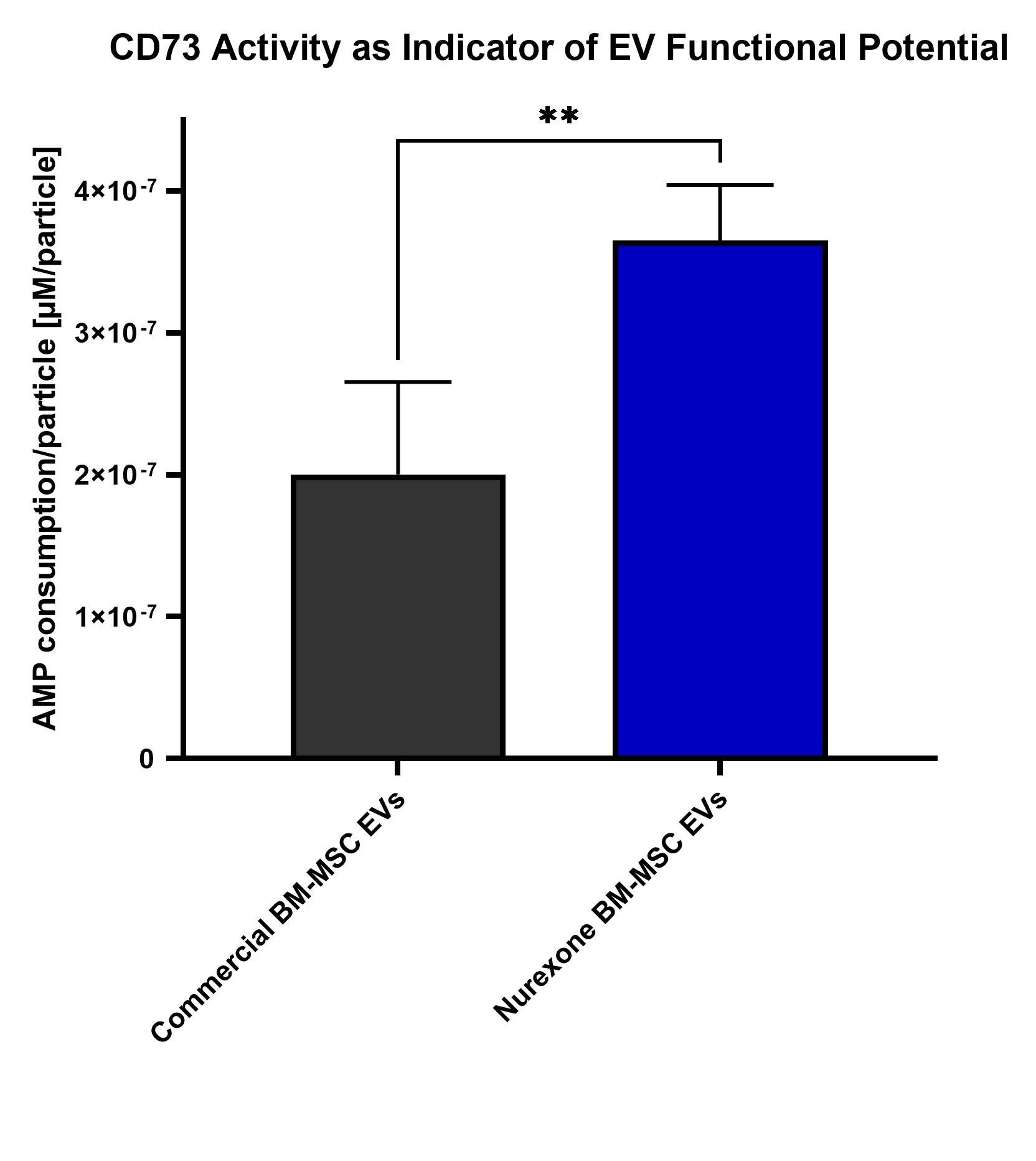
Recent analytical testing demonstrated that NurExone's exosomes produced from human bone marrow–derived mesenchymal stem cells (" MSC ") exhibit significantly higher CD73-associated biological activity compared with a commercially available MSC exosome control. CD73 is a surface enzyme linked to extracellular signaling and tissue-repair mechanisms.
As shown in the graph below, the average of NurExone's two manufacturing batches demonstrated meaningfully higher adenosine monophosphate (" AMP ") to adenosine conversion per particle compared with the control. The difference was statistically significant (t-test, p < 0.01), indicating that NurExone's exosomes possess stronger functional characteristics associated with regenerative potential, or in other words NurExone's exosomes can send stronger "healing" or "anti-inflammatory" signals.
These findings validate the potency of NurExone's therapeutic exosomes strengthening the biological rationale for their use as a delivery vehicle in ExoPTEN.
These results demonstrate the high quality of the Master Cell Bank acquired by NurExone and the knowhow held by NurExone and its patented 3D shear-force production technology, indicating that the manufacturing approach described in the patent can yield higher-quality, more potent exosomes in practice.
Engagement of Russo Partners
Subject to TSX Venture Exchange (" TSXV ") approval, NurExone has engaged Russo Partners LLC, a New York–based strategic communications firm, (" Russo ") for an initial consulting project for up to two months for a fixed fee of US$6,600.
Either party may terminate the engagement with 30 days' notice. Russo does not currently have a direct or indirect interest in the securities of the Company. While Russo has no intention of acquiring any additional securities of the Company at this time, it may do so in the future in compliance with applicable securities laws and TSXV policies.
About Russo Partners
Russo Partners is a strategic communications firm focused on innovators in healthcare and technology. Founded as Noonan/Russo in 1988, the firm collaborates on integrated communications programs for biopharma, MedTech, diagnostics, healthcare IT and healthcare services companies worldwide. The firm's team is comprised of Ph.D.s, M.D.s., former journalists and members of Wall Street. Russo Partners is known for delivering award-winning strategic counsel and services aligned with its core values of passion, trust, forward-thinking and client focus. For more information, visit www.russopartnersllc.com .
About NurExone
Nurexone Biologic Inc. is a TSXV, OTCQB, and Frankfurt-listed biotech company focused on developing regenerative exosome-based therapies for central nervous system injuries. Its lead product, ExoPTEN, has demonstrated strong preclinical data supporting clinical potential in treating acute spinal cord and optic nerve injury, both multi-billion-dollar markets i . Regulatory milestones, including obtaining the Orphan Drug Designation, facilitates the roadmap towards clinical trials in the U.S. and Europe. Commercially, the Company is expected to offer solutions to companies interested in quality exosomes and minimally invasive targeted delivery systems for other indications. NurExone has established Exo-Top Inc., a U.S. subsidiary, to anchor its North American activity and growth strategy.
For additional information and a brief interview, please watch Who is NurExone? , visit www.nurexone.com or follow NurExone on LinkedIn , Twitter , Facebook , or YouTube .
For more information, please contact:
Dr. Lior Shaltiel
Chief Executive Officer and Director
Phone: +972-52-4803034
Email: info@nurexone.com
Dr. Eva Reuter
Investor Relations – Germany
Phone: +49-69-1532-5857
Email: e.reuter@dr-reuter.eu
Allele Capital Partners
Investor Relations – U.S.
Phone: +1 978-857-5075
Email: aeriksen@allelecapital.com
FORWARD-LOOKING STATEMENTS
This press release contains certain "forward-looking statements" that reflect the Company's current expectations and projections about its future results. Wherever possible, words such as "may", "will", "should", "could", "expect", "plan", "intend", "anticipate", "believe", "estimate", "predict" or "potential" or the negative or other variations of these words, or similar words or phrases, have been used to identify these forward-looking statements. Forward-looking statements in this press release include, but are not limited to, statements relating to: the Company advancing ExoPTEN's manufacturing processes and analytical methods; the Company's evaluating potential Israeli production partners and organizations for early, small ‑ scale GMP ‑ aligned manufacturing runs of ExoPTEN; the Company's preparing for compassionate-use filings once regulatory pathways permit; the Company launching a first compassionate-use and first-in-human programs; expectations associated with the Master Cell Bank and the Company's patented 3D shear-force production technology; the Company engaging Russo; the Company preparing regulatory submissions; receipt of regulatory approvals, including TSXV approval; and the NurExone platform technology offering novel solutions to drug companies interested in minimally invasive targeted drug delivery for other indications.
These statements reflect management's current beliefs and are based on information currently available to management as at the date hereof. In developing the forward-looking statements in this press release, we have applied several material assumptions, including: the Company has the ability to advance ExoPTEN's manufacturing processes and analytical methods; the Company will prepare regulatory submissions; suitable Israeli manufacturing partners with requisite GMP capabilities will be identified and engaged on acceptable terms; early, small clinical grade batches can and will be produced, characterized and validated in alignment with future regulatory requirements; applicable compassionate-use or expanded-access pathways will be available and that regulatory agencies will accept the Company's filings; the Master Cell Bank will continue to meet quality, consistency and supply requirements; the patented production approach will operate as intended at planned scales; the engagement of Russo will receive TSXV approval and the consulting services will benefit the Company; the Company has the ability to launch a first compassionate-use and first-in-human programs; and the NurExone platform technology has the ability to offer novel solutions to drug companies interested in minimally invasive targeted drug delivery for other indications.
Forward-looking statements involve significant risk, uncertainties and assumptions. Many factors could cause actual results, performance or achievements to differ materially from the results discussed or implied in the forward-looking statements. These risks and uncertainties include, but are not limited to risks related to: the Company's early stage of development; lack of revenues to date; the inherent uncertainty of preclinical drug development, including challenges in identifying, engaging or retaining qualified manufacturing partners, achieving and maintaining GMP compliance, and producing consistent batches of ExoPTEN; the Company will not file for compassionate-use; the Master Cell Bank will no longer meet quality, consistency and/or supply requirements; the engagement of Russo will not be approved; the Company will not yield the intended benefits from their engagement of Russo; the risk that product candidates may not advance to clinical trials or receive regulatory approval; the possibility that results from preclinical studies and early-stage trials may not predict later outcomes; the uncertain timing, cost, and outcome of preclinical and clinical development activities; risks related to the clinical trial process, including potential delays or failure to achieve effective trial design or positive results; the inability to obtain or maintain required regulatory approvals; limited market acceptance of the Company's products, even if approved; the potential emergence of competing therapies that are safer, more effective, or more affordable; rapid technological change that may impact the relevance of the Company's technologies; the Company's dependence on key personnel and strategic partners; the inability to obtain adequate financing; risks related to the Company's ability to protect its intellectual property; the possibility that the Company's technologies, including its exosome-based platforms, may not achieve their intended therapeutic impact; the inability to produce or scale exosome-based products for clinical use; limited adoption in regenerative medicine or cell therapy applications; lack of growing clinical demand in targeted indications such as spinal cord injury, optic nerve repair, or other therapeutic areas; failure to meet planned development milestones or achieve commercial breakthroughs; the Company will not advance the ExoPTEN's manufacturing processes and/or analytical methods; the Company will not prepare regulatory submissions; the Company will not launch first compassionate-use nor first-in-human programs; the NurExone platform technology not offering novel solutions to drug companies interested in minimally invasive targeted drug delivery for other indications; and the risks discussed under the heading "Risk Factors" on pages 44 to 51 of the Company's Annual Information Form dated August 27, 2024, a copy of which is available under the Company's SEDAR+ profile at www.sedarplus.ca . These factors should be considered carefully, and readers should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in this press release are based upon what management believes to be reasonable assumptions, the Company cannot assure readers that actual results will be consistent with these forward-looking statements. These forward-looking statements are made as of the date of this press release, and the Company assumes no obligation to update or revise them to reflect new events or circumstances, except as required by law.
Neither TSXV nor its Regulation Services Provider (as that term is defined in the policies of the TSXV) accepts responsibility for the adequacy or accuracy of this release.
i Spinal cord injury , Glaucoma
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/d5b187f6-3be5-4601-a83d-d94953a35a13