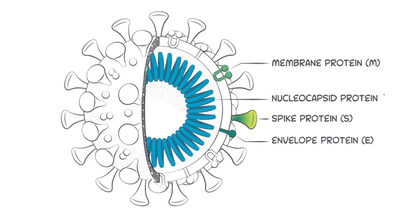
Beckman Coulter, a clinical diagnostics leader, today announced its Access SARS-CoV-2 Immunoglobulin M (IgM) assay has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA). The assay detects antibodies that recognize the receptor binding domain (RBD) of the spike protein which the SARS-CoV-2 virus uses to enter the human host cells. The assay, which demonstrates 99.9% specificity and 98.3% sensitivity, is part of a suite of diagnostic solutions being developed by Beckman Coulter in response to the ongoing COVID-19 pandemic to guide physicians and patients in their healthcare decision making.
Beckman Coulter's Access SARS-CoV-2 IgM antibody assay receives Emergency Use Authorization from the U.S. FDA
"Since March, the Beckman Coulter team has worked around the clock to develop a suite of assays that play a critical role in the ongoing global fight against COVID-19," said Julie Sawyer Montgomery , president of Beckman Coulter. "As a science-driven company, we continue in our commitment to deliver rigorously validated diagnostics of the highest quality that provide meaningful information, so doctors and patients alike can trust the results for urgent, care decisions."
Beckman Coulter's suite of COVID-19 testing solutions includes the Access SARS-CoV-2 IgM assay and the Access SARS CoV-2 IgG assay, which received EUA in June. The company also recently received FDA Emergency Use Authorization for its interleukin 6 (IL-6) assay, which can be used to assist in identifying severe inflammatory response in patients with confirmed COVID 19 illness to aid in determining the risk of intubation with mechanical ventilation, in conjunction with clinical findings and the results of other laboratory testing. Beckman Coulter is also currently developing a SARS CoV-2 antigen assay as well as a quantitative IgG assay anticipated to be launched later this year.
All of the Beckman Coulter assays to address COVID-19 can be performed in automated or high-throughput immunoassay formats, as well as Beckman Coulter's Access 2 analyzer, a compact, table-top analyzer enabling high-quality serology testing to be carried out in small hospitals and clinics. Additionally, all of the assays seamlessly integrate into laboratory workflows making it easy to add these tests to routine blood tests performed during inpatient and wellness testing.
For more information on Beckman Coulter's suite of testing solutions or its commitment to the fight against COVID-19, visit: www.BeckmanCoulter.com/Coronavirus .
About the Access SARS-CoV-2 IgM Assay
The Access SARS-CoV-2 IgM Assay is a qualitative immunoassay that detects IgM antibodies. The test has confirmed 98.3% positive percent agreement (sensitivity) at 15-30 days post symptom onset and 99.9% negative percent agreement (specificity). The assay utilizes an immunocapture format to bind patient IgM antibodies on the magnetic particle solid phase and a recombinant SARS-CoV-2 protein - enzyme conjugate to detect anti-SARS-CoV-2 IgM. The Access SARS-CoV-2 IgM assay can be used in Random Access Mode (RAM), which means that the antibody tests can be run along with other immunoassay tests. The assay can also be used with a variety of Beckman Coulter analyzers, including the high-throughput DxI 800 designed for large labs, to the DxI 600 for mid-sized labs and the DxC 600i and Access 2 analyzers for smaller labs and healthcare clinics.
About Beckman Coulter
Beckman Coulter is committed to advancing healthcare for every person by applying the power of science, technology and the passion and creativity of our teams to enhance the diagnostic laboratory's role in improving healthcare outcomes. Our diagnostic systems are used in complex biomedical testing, and are found in hospitals, reference laboratories and physician office settings around the globe. Beckman Coulter offers a unique combination of people, processes and solutions designed to elevate the performance of clinical laboratories and healthcare networks. We do this by accelerating care with a menu that matters, bringing the benefit of automation to all, delivering greater insights through clinical informatics and unlocking hidden value through performance partnership. An operating company of Danaher Corporation (NYSE: DHR) since 2011, Beckman Coulter is headquartered in Brea, Calif. , and has more than 11,000 global associates working diligently to make the world a healthier place.
© 2020 Beckman Coulter. All rights reserved. Beckman Coulter, the stylized logo, and the Beckman Coulter product and service marks mentioned herein are trademarks or registered trademarks of Beckman Coulter, Inc. in the United States and other countries.
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/beckman-coulter-sars-cov-2-igm-antibody-test-receives-fda-emergency-use-authorization-301149222.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/beckman-coulter-sars-cov-2-igm-antibody-test-receives-fda-emergency-use-authorization-301149222.html
SOURCE Beckman Coulter Diagnostics