Top 4 NASDAQ Genetics Stocks (Updated January 2026)
Overview
Mental health and trauma impact a significant amount of adults in the United States, with 52.9 million adults experiencing a mental illness such as severe forms of anxiety with 12 million adults experiencing post-traumatic stress disorder (PTSD). Unfortunately, treatment for mental illnesses is often ineffective, especially for trauma-based conditions. Psychedelics have emerged as a promising treatment for many mental illnesses, and two-thirds of physicians believe one psychedelic, psilocybin, has therapeutic benefits.
Several neurological disorders, such as brain trauma, have shown promising results from psilocybin treatment. Mental illnesses such as major depression have also indicated encouraging results from psilocybin. Psychedelic compounds are generally considered non-addictive and suitable for the treatment of a wide variety of medical conditions. Companies that innovate safe and effective psychedelic medications are leading the charge toward a new era of mental health treatment.
Lobe Sciences (CSE:LOBE,OTCQB: LOBEF) is a Canadian-based life sciences company entering clinical stage development for proprietary, patent-pending psilocin compounds to treat neurological and brain diseases. Psilocin is known to be the psychologically active component of psilocybin. In 2022 the company announced it is entering human clinical trials for its proprietary psilocin compound L-130. The company is positioned to initiate additional clinical trials in Australia and plans to file an investigational new drug application (IND) with the US FDA in 2023.
Prior to the work performed at Lobe Sciences, it was generally believed that the psilocin molecule could not be stabilized outside nature’s “prodrug” psilocybin. Lobe Sciences has developed multiple novel stable psilocin compounds. The L-130, for example, is a stabilized and patent-pending form of psilocin. Lobe Sciences received regulatory clearance to conduct Phase 1 study which is a combination of safety and pharmacokinetics evaluation of a fixed dose of L-130.
The company's exclusive discovery and manufacturing partner Quality Chemical Laboratories LLC (QCL) initiated commercial formulation activities for L-130. QCL, in collaboration with Clearway Global, LLC, will prepare the chemical, manufacturing and control section of the investigational new drug application. The material produced at QCL will be used in the recently announced Phase IIIa study to evaluate L-130 as a treatment for chronic cluster headaches, a debilitating orphan disease. QCL agrees to supply Lobe Sciences research and commercial quantities of two new chemical entities, L-130 and L-131 under an exclusive arrangement.
Lobe Sciences’ vision is to administer psilocin compounds at home, in the emergency department, or clinic. This will allow patients to remain with their primary physician and not be required to be confined in a clinic for eight or more hours to receive a hallucinatory dose of the psychedelic. The company plans to develop psilocin-based therapeutics for the treatment of neurologic disorders, such as severe anxiety and PTSD. Research data from the company indicates that its combination therapeutic method is significantly more effective than monotherapy for both mTBI and PTSD.
The first focus is on identifying the largest non-hallucinatory dose of psilocin for treating neurologic and brain diseases. The company anticipates that it will file an investigational new drug (IND) in early 2023.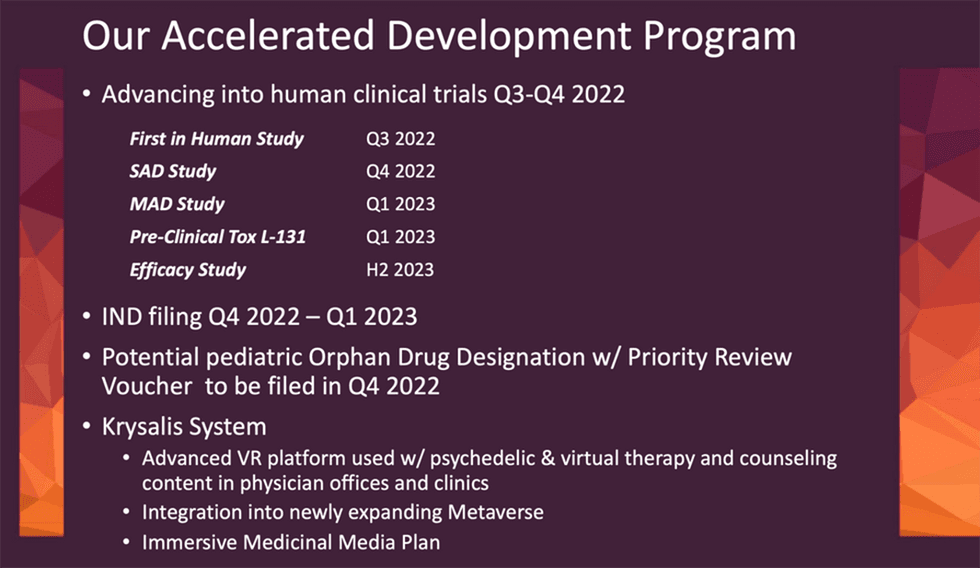
Company Highlights
- Lobe Sciences is a life sciences company headquartered in Canada with North American operations.
- The company is entering clinical-stage development for proprietary patent-pending psilocin compounds, developing psychedelic therapeutics as an integrated biotech company.
- Lobe Sciences’ therapeutic development is focused on neurologic and brain diseases. Recent pre-clinical data has demonstrated the effectiveness of these treatments.
- Provisional patents for the Preparation of Stable Psilocin Prodrugs and Analogues and their Uses
- Lobe Sciences has partnered with Clearway Global to assist with developing, implementing and monitoring its manufacturing, regulatory and clinical programs.
- Lobe has completed the synthesis of bulk L-130 (a molecular modification of naturally occurring psilocin) and of the clinical supplies to be used in upcoming trials. Sufficient quantities of L-130 have been manufactured for the company’s first two Phase 1 programs.
- Lobe Sciences filed a provisional patent for the preparation and use of its proprietary and stable psilocin-related compounds.
Get access to more exclusive Psychedelics Investing Stock profiles here