June 12, 2024
The Board of BPH Global Ltd (ASX: BP8) (Company) is pleased to announce that the Company has further reviewed and refined its R&D program, and in particular, its Seaweed-sourced energy R&D program.
Highlights
- Key features of the R&D program:
- Fermentation: Harvested seaweed to be processed by fermentation (Anaerobic Digestion).
- Biohydrogen Extraction: Production of biohydrogen/biogases as a natural by-product of fermentation.
- Essential Mineral and Nutraceutical extraction: Fermentation to unlock essential minerals and nutraceuticals.
- Artificial Intelligence technology (AI): AI to be developed and deployed to enhance essential mineral and nutraceutical identification and extraction.
- Commercialisation: R&D focussed on potential commercialisation opportunities.
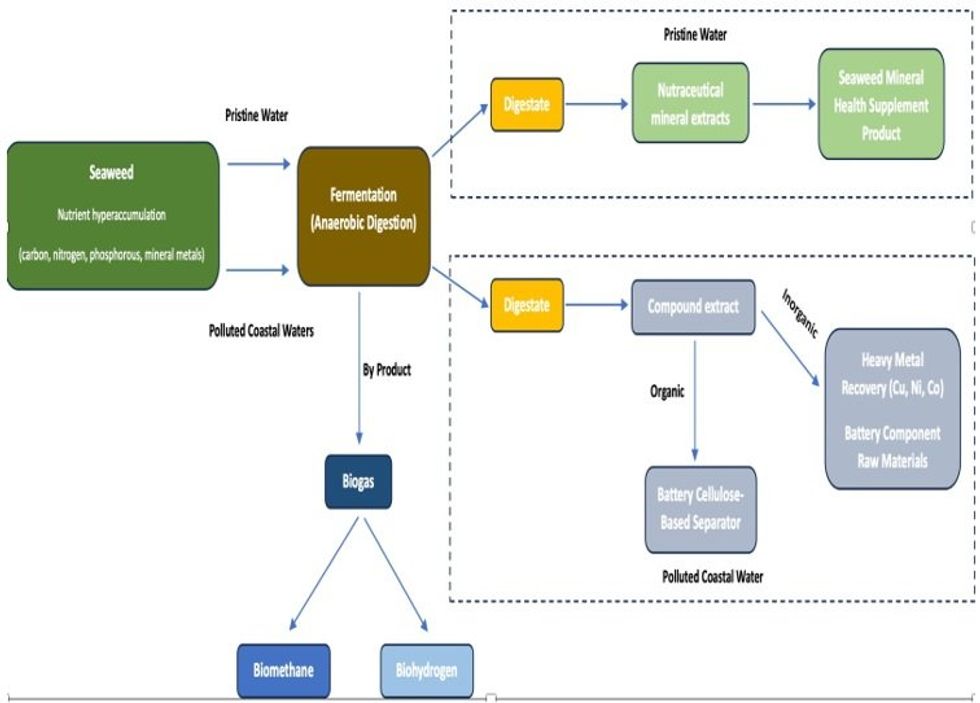
The Company had previously announced that it was expanding its R&D program to include R&D into the extraction of individual macro minerals and micro minerals from seaweed and sea plant biomass, to enable the sale of those macro and micro minerals to the commodities markets, and to industry specific markets such as the battery industry, and thereby create additional revenue streams for the Company. In that same announcement, the Company also stated that it would investigate both pyrolytic and non- pyrolytic/low heat extractive strategies to determine the highest yield of targeted essential minerals and chemical compounds out of selected seaweed species.
Since that announcement, the Company has continued to review and refine its R&D program. In doing so, the Company has considered factors such as environmental impacts and benefits; development costs; prospects of achieving proof of concept; and the likelihood of successful commercialisation. The Company has decided to focus its R&D on the non-pyrolytic/low heat process of fermentation (Anaerobic Digestion) as its preferred extractive strategy. The process of fermentation produces an intermediate liquid product from which essential minerals (and nutraceuticals) can be extracted. In addition, a by-product of that fermentation process is the production of biogases, principally biohydrogen and biomethane.
Consequently, the Company has decided it will now focus its energy related R&D program on these two related activities:
- Production and extraction of biohydrogen/biogases; and
- Extraction of essential minerals.
1. Biohydrogen/biogas production
Production of biohydrogen/biogas a natural by-product of fermentation: The process of fermentation (Anaerobic Digestion) transforms seaweed biomass to an intermediate liquid product from which essential minerals (and nutraceuticals) can be extracted. A by-product of that fermentation process is the production of biogases, principally biohydrogen and biomethane. A major source of renewable energy, biogas is created by the breakdown of organic matter in the absence of oxygen. It is produced by the anaerobic digestion of various organic materials including municipal waste, farm waste, food waste and energy crops. The Company will undertake a R&D program on the production of biogases from seaweed as a by- product of the extraction of essential minerals and nutraceuticals from seaweed. Biogas can be stored in tanks and transported. Biodigesters reduce methane emissions, making biogas a smart and valuable climate and clean air energy solution.
Illustration of the Company’s R&D Program regarding the extraction of essential minerals from seaweed using the fermentation extractive method and the production of biogases (biohydrogen and biomethane) as a by-product
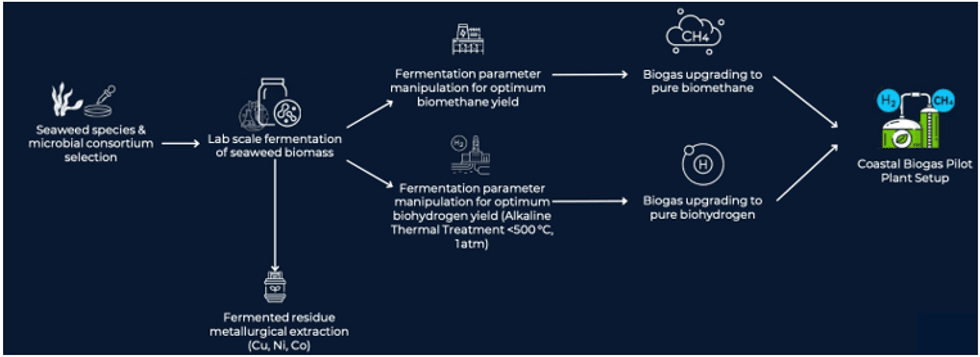
- Proposed R&D program and pathway to commercialisation: The Company’s R&D program regarding the production of biogases from seaweed will focus on the:
- production of pure biohydrogen and biomethane through a process of upgrading the biohydrogen and biomethane yields obtained from the fermentation process;
- use of pure biohydrogen and pure biomethane as the fuel source for a coastal biogas pilot plant; and
- sale of biohydrogen and biomethane to energy companies.
- Biogas Genset System: Biogas can be used as a fuel to generate electricity using a small scale genset. Biogas is converted to mechanical energy through an internal combustion engine. The mechanical energy rotates an electric generator which produces the electricity.
Click here for the full ASX Release
This article includes content from BPH Global Ltd, licensed for the purpose of publishing on Investing News Australia. This article does not constitute financial product advice. It is your responsibility to perform proper due diligence before acting upon any information provided here. Please refer to our full disclaimer here.
BP8:AU
The Conversation (0)
01 December 2021
BPH Global
Stemcell United Ltd is a marine and agricultural biotechnology industry. Its only operating segment being sourcing, producing, marketing, and selling traditional medicines.
Stemcell United Ltd is a marine and agricultural biotechnology industry. Its only operating segment being sourcing, producing, marketing, and selling traditional medicines. Keep Reading...
20 February 2025
Exceptional silver and cobalt assays from seaweed
BPH Global (BP8:AU) has announced Exceptional silver and cobalt assays from seaweedDownload the PDF here. Keep Reading...
31 January 2025
Quarterly Activities/Appendix 4C Cash Flow Report
BPH Global (BP8:AU) has announced Quarterly Activities/Appendix 4C Cash Flow ReportDownload the PDF here. Keep Reading...
22 January 2025
Completion of Indonesian Seaweed Joint Venture Transaction
BPH Global (BP8:AU) has announced Completion of Indonesian Seaweed Joint Venture TransactionDownload the PDF here. Keep Reading...
17 December 2024
BPH Global receives funding commitment of A$100,000
BPH Global (BP8:AU) has announced BPH Global receives funding commitment of A$100,000Download the PDF here. Keep Reading...
15 December 2024
Private Placement
BPH Global (BP8:AU) has announced Private PlacementDownload the PDF here. Keep Reading...
Latest News
Interactive Chart
Latest Press Releases
Related News
TOP STOCKS
American Battery4.030.24
Aion Therapeutic0.10-0.01
Cybin Corp2.140.00