
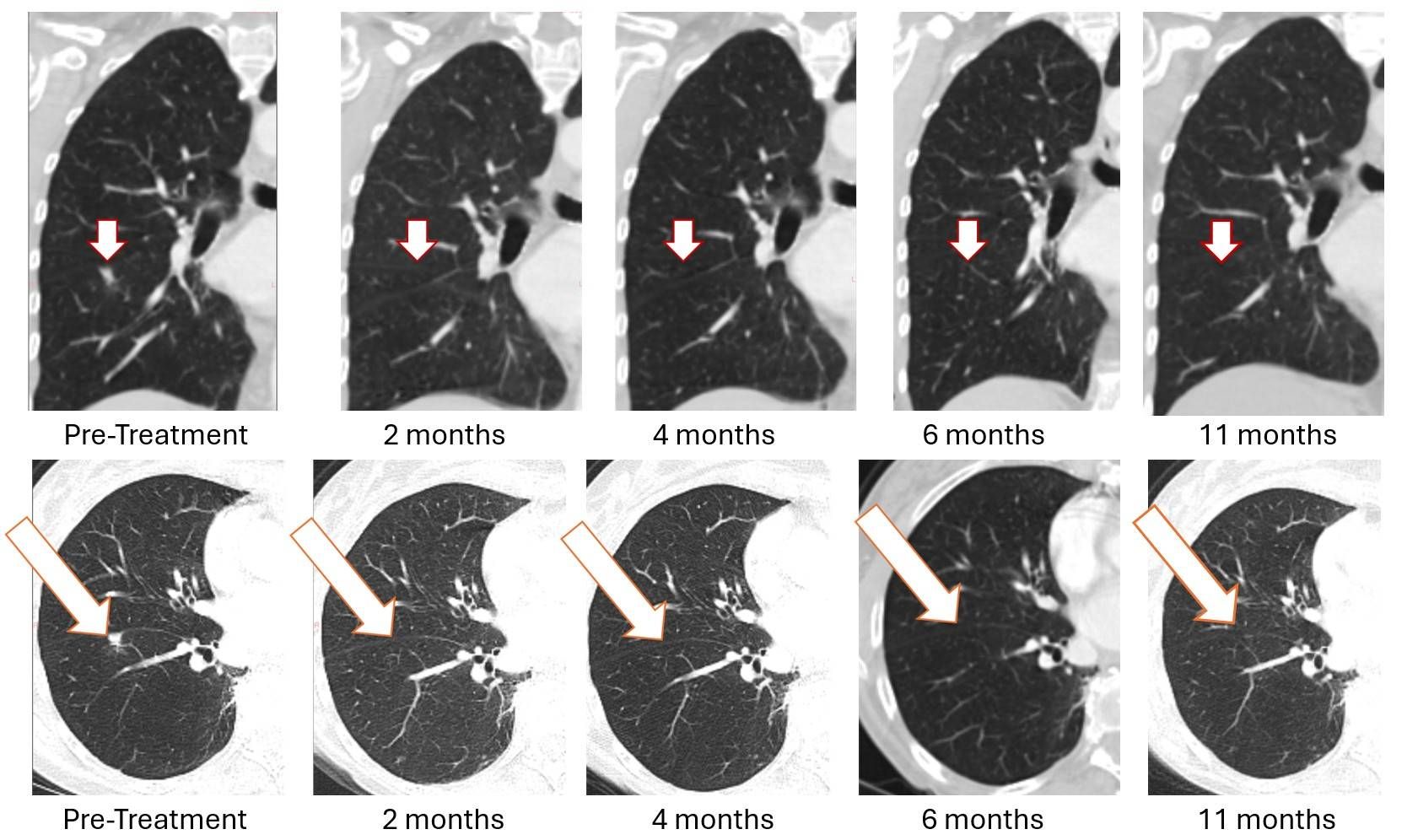
- [IMAGES BELOW] 11-month sustained complete resolution of lung metastasis observed in Bria-OTS Phase 1/2a metastatic breast cancer study.
- No treatment limiting toxicities reported.
- Patient maintained stable disease at all other evaluable sites.
briacell therapeutics corp. (Nasdaq: BCTX, BCTXW, BCTXZ) (TSX: BCT) ("BriaCell" or the "Company"), a clinical-stage biotechnology company developing novel immunotherapies to transform cancer care, today announces the durable and sustained complete resolution of a lung metastasis in a patient with metastatic breast cancer (MBC) treated with Bria-OTS, BriaCell's personalized off the shelf immunotherapy. The patient is hormone receptor-positive (HR+), HER2-negative.
The first patient enrolled in the Bria-OTS study, a 78-year-old woman with advanced metastatic breast cancer and multiple prior treatment failures, achieved complete (100%) resolution of a lung metastasis following four doses of Bria-OTS single agent therapy. The complete response, initially observed at 2 months (previously reported), was subsequently confirmed at 4 months (previously reported), 6 months (previously reported), and now at 11 months. The patient received 17 cycles of Bria-OTS, completed 12 months of the study, and remains in survival follow-up.
Figure 1: Treatment with Bria-OTS monotherapy resulted in 100% resolution of tumor in the right lung of the metastatic breast cancer (MBC) patient following 2 months of therapy and confirmed at 4, 6, and 11 months of therapy1 (axial and coronal views)
The Phase 1 dose escalation portion of the study is complete and the Phase 2a portion, evaluating combination of Bria-OTS with an immune checkpoint inhibitor, is now underway.
"The sustained clinical response observed in this late-stage MBC patient, who had previously progressed through multiple prior treatments is remarkable," stated Neal S. Chawla MD, Director at the Sarcoma Oncology Center, Santa Monica, CA, and Principal Investigator for the Bria-OTS study. "We are excited to further evaluate Bria-OTS in combination with an immune checkpoint inhibitor in metastatic breast cancer."
"These clinical findings reinforce our confidence in the therapeutic potential and safety of the Bria-OTS platform," added Dr. William V. Williams, BriaCell's President and CEO. "Our team remains committed to advancing our novel therapeutic approach with the goal of making a meaningful difference for patients with metastatic breast cancer, particularly those with limited treatment options."
About Bria-OTS
Bria-OTS is a next generation, off-the-shelf personalized immunotherapy based on BriaCell's lead candidate Bria-IMT currently being evaluated in a Phase 1/2a study (ClinicalTrials.gov identifier: NCT06471673) in patients with metastatic recurrent breast cancer. The trial includes both monotherapy dose escalation and checkpoint inhibitor combination dose expansion cohorts. The Company has recently entered the dose expansion phase.
About briacell therapeutics corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/.
Safe Harbor
This press release contains "forward-looking statements" that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release include statements regarding: BriaCell continuing the Phase 1/2a Bria-OTS study and reproducing similar results in patients with MBC and other cancers; the use of the Bria-OTS platform as monotherapy; and Bria-OTS's validation as a personalized immunotherapy approach. Forward-looking statements may be identified by the use of words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "aim," "should," "will," "would," or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading "Risks and Uncertainties" in the Company's most recent Management's Discussion and Analysis, under the heading "Risk Factors" in the Company's most recent Annual Information Form, and under "Risks and Uncertainties" in the Company's other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company's profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and briacell therapeutics corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:
William V. Williams, MD
President & CEO
1-888-485-6340
info@briacell.com
Investor Relations Contact:
investors@briacell.com
1 Note that the other white dots in the lungs are blood vessels.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/be299ddd-4e02-4bf0-8b5a-f704f172076e




