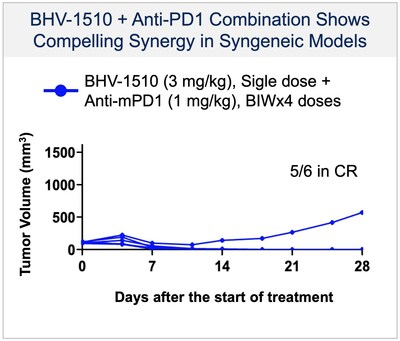
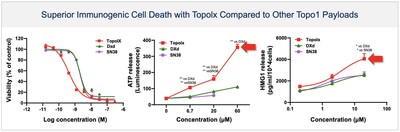
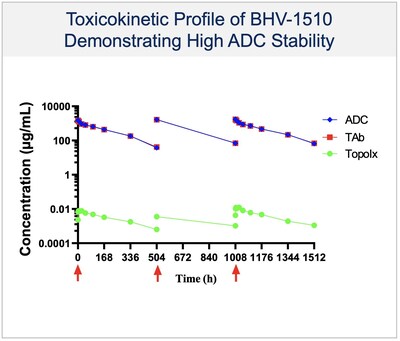
-BHV-1510 is a novel trophoblast cell surface antigen-2 (Trop-2) directed antibody drug conjugate (ADC) that has demonstrated a highly differentiated preclinical monotherapy efficacy profile, the potential for broader therapeutic margin than other Trop-2 ADCs currently in development, and synergistic affects when combined with anti-PD1 therapy
-Biohaven also entered into a clinical supply agreement with Regeneron to study the combination of BHV-1510 with Regeneron's anti-PD-1 Libtayo ® (cemiplimab-rwlc) in the Phase 1/2 clinical trial
-Malignancies of epithelial tissue account for the vast majority of all cancers and the advanced or metastatic forms of these carcinomas represent an urgent unmet medical need
Biohaven Ltd. (NYSE: BHVN) (Biohaven), a global clinical-stage biopharmaceutical company focused on the discovery, development and commercialization of life-changing therapies to treat a broad range of rare and common diseases, announced the first patient has been dosed in a first-in-human Phase 12 study of BHV-1510, a highly differentiated Trophoblast Cell Surface Antigen-2 (Trop-2) directed Antibody Drug Conjugate (ADC), and the lead ADC program to advance into clinical trials in Biohaven's growing oncology pipeline.
The Phase 1/2 study of BHV-1510 is a multicenter, open-label study in subjects with select advanced or metastatic epithelial cell tumors. The trial consists of a dose-escalation phase, followed by a multicohort expansion phase. Additional information can be found at https://www.clinicaltrials.gov/ (NCT06384807).
"We are extremely proud to advance our first oncology clinical program with a potentially best in class ADC," said Nushmia Khokhar , M.D., Chief Medical Officer of Oncology at Biohaven. "With the initiation of this monotherapy study, we are one step closer to providing differentiated and superior treatment options to people living with cancer. We are also excited to work with Regeneron and efficiently explore BHV-1510 in combination with its PD-1 inhibitor Libtayo ® across a range of tumors."
BHV-1510 is a next-generation, fully optimized ADC that consists of a Trop-2 directed antibody conjugated to a proprietary best-in-class Topoisomerase 1 (TopoIx) payload at a homogeneous drug-antibody ratio (DAR) of 4. BHV-1510 incorporates a unique site-specific conjugation methodology and highly stable and irreversible linker chemistry designed by GeneQuantum Healthcare Co. (Suzhou) Ltd. Preclinically BHV-1510 has shown superior cellular cytotoxicity, bystander killing, and immunogenic cell death resulting in improved efficacy as monotherapy, and synergistic efficacy in combination with anti-PD-1 therapy. In IND-enabling studies, BHV-1510 also showed a broader therapeutic margin relative to more advanced Trop-2 ADCs, including a lack of lung toxicity, that may translate to an improved clinical efficacy and safety profile.
Shiraj Sen M.D ., Ph.D., Director of NEXT Oncology-Dallas, commented, "Antibody drug conjugates have shown promising efficacy in solid tumors, but their clinical potential is currently limited by their safety margin. BHV-1510 has compelling and differentiated preclinical data, with the potential to translate to better safety and efficacy in several tumors including those with significant unmet need. We are excited to be working with the Biohaven team on this important clinical trial for patients with advanced epithelial tumors."
Biohaven entered into a clinical supply agreement with Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN) under which Biohaven will sponsor and fund the planned clinical trial and Regeneron will provide Libtayo. Libtayo is a fully-human monoclonal antibody targeting the immune checkpoint receptor PD-1 (programmed cell death protein-1).
Brian Lestini , M.D., Ph.D., President of Oncology at Biohaven stated, "Biohaven's ADC technology and portfolio has the potential to differentiate from current ADCs in the market that use older payloads or maleimide linkers. The advancement of our lead ADC program, BHV-1510, into clinic as both monotherapy and in combination with Regeneron's anti-PD-1 represents an important first opportunity to show the breadth and differentiation of Biohaven's extensive ADC pipeline. Coupled with our deep in-house expertise in oncology clinical development, ADC chemistry, and complex manufacturing, we believe our diverse and growing ADC portfolio positions Biohaven for future leadership in oncology."
Biohaven is developing a broad portfolio of highly differentiated ADCs with the potential to broaden therapeutic margin, increase time on treatment, and improve efficacy. Biohaven's proprietary MATE™ platform technology focuses on novel, single-step conjugation chemistry, with the potential to be superior to the current industry standard maleimide and lipophilic click chemistry.
About Biohaven
Biohaven is a biopharmaceutical company focused on the discovery, development, and commercialization of life-changing treatments in key therapeutic areas, including immunology, neuroscience, and oncology. The company is advancing its innovative portfolio of therapeutics, leveraging its proven drug development experience and multiple proprietary drug development platforms. Biohaven's extensive clinical and preclinical programs include Kv7 ion channel modulation for epilepsy and mood disorders; extracellular protein degradation for immunological diseases; TRPM3 antagonism for migraine and neuropathic pain; TYK2/JAK1 inhibition for neuroinflammatory disorders; glutamate modulation for OCD and SCA (spinocerebellar ataxia); myostatin inhibition for neuromuscular and metabolic diseases, including SMA and obesity; antibody recruiting bispecific molecules and antibody drug conjugates for cancer. For more information, visit www.biohaven.com .
Forward-looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including "continue", "plan", "will", "believe", "may", "expect", "anticipate" and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of development candidates, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials; the timing of planned interactions and filings with the FDA; the timing and outcome of expected regulatory filings; complying with applicable U.S. regulatory requirements; the potential commercialization of Biohaven's product candidates; the potential for Biohaven's product candidates to be first in class therapies; and the effectiveness and safety of Biohaven's product candidates. Additional important factors to be considered in connection with forward-looking statements are described in Biohaven's filings with the Securities and Exchange Commission, including within the sections titled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations". The forward-looking statements are made as of the date of this news release, and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
MATE™ is a trademark of Biohaven Therapeutics Ltd.
Libtayo® is a registered trademark of Regeneron Pharmaceuticals, Inc.
Investor Contact:
Jennifer Porcelli
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
+1 (201) 248-0741
Media Contact:
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
+1 (312) 961-2502
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/biohaven-doses-first-patient-with-its-novel-trop-2-directed-antibody-drug-conjugate-adc-bhv-1510-in-advanced-or-metastatic-epithelial-tumors-302157427.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/biohaven-doses-first-patient-with-its-novel-trop-2-directed-antibody-drug-conjugate-adc-bhv-1510-in-advanced-or-metastatic-epithelial-tumors-302157427.html
SOURCE Biohaven Ltd.