In the news release, BioHarvest Sciences Inc.'s First Cannabis Cell Reservoir Produces Ongoing Flowering Stage Cannabinoid Cells For 2 Years, issued 07-Jul-2021 by BioHarvest Sciences Inc. over PR Newswire, we are advised by the company that an incorrect version of the release was issued inadvertently. The complete, corrected release follows:
BioHarvest Sciences Inc.'s First Cannabis Cell Reservoir Produces Ongoing Flowering Stage Cannabinoid Cells For 2 Years
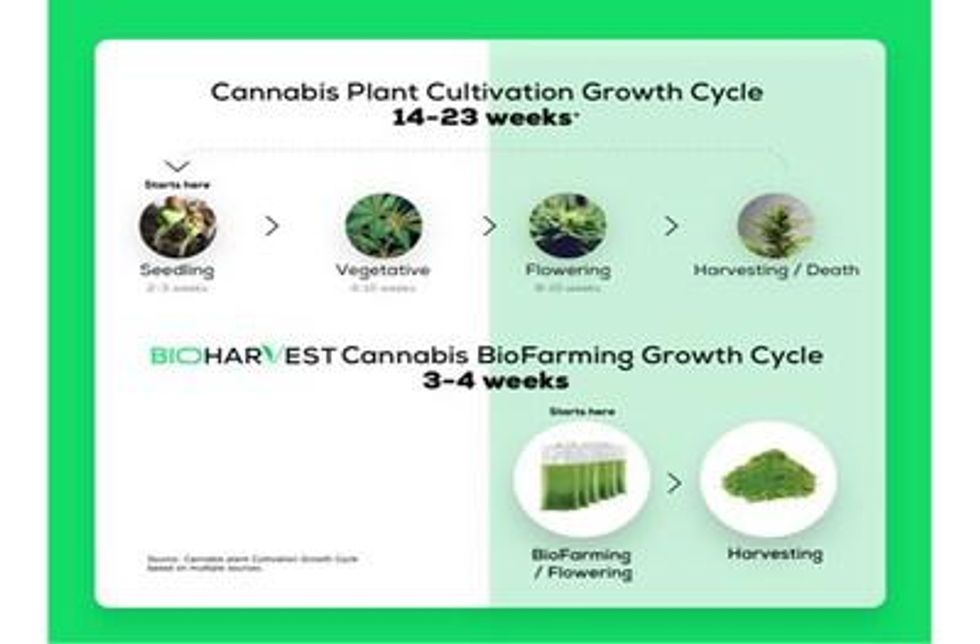
- The Company demonstrates the efficiency and reliability of its BioFarming technology by reducing production cycle time for Cannabis from 14-23 weeks to 3-4 weeks.
- The Company's BioFarming technology will allow the Company to harvest 13-17 cycles per year versus an average of 4 cycles per year for conventional Cannabis cultivation.
- The Company is on track to reach commercialization in H1/2022.
VANCOUVER, BC and REHOVOT, Israel , July 7, 2021 /PRNewswire/ -- BioHarvest Sciences Inc. ("BioHarvest" or the "Company") (CSE: BHSC) today announces it has reached a significant milestone in its production of Cannabinoids, with its first cell reservoir producing Cannabis Trichomes (the natural micro-factories producing Cannabinoids) for the past two years. This achievement demonstrates the efficiency and reliability of the Company's BioFarming technology to produce the "flowering" stage of the Cannabis growth cycle at scale, which is significantly shorter, more productive, and cost-efficient, and more environmentally sustainable than conventional Cannabis cultivation.
BioHarvest's ground-breaking technology employs the original Cannabis plant and its respective cells as starting material only once, allowing the Company to harvest 13-17 cycles per year versus an average of 4 cycles per year for conventional Cannabis cultivation. The Company's bioreactors can reliably and continuously produce Cannabis cells without the need to source the mother plant, seeds, or any other starting material again. This efficiency results from bypassing the "seeding" and "vegetative" stage, usually taking a Cannabis plant anywhere between 6 and 13 weeks.
In addition, BioHarvest's Cannabis cells are able to remain in the flowering stage continuously, in contrast to a typical Cannabis flower that will terminate after 9 or 10 weeks of flowering and will be immediately followed by the end of the plant's life cycle. In that case, new starting material will need to be sourced and propagated, and new seedlings will be required to be grown through the vegetative stage, once again. BioHarvest's BioFarming technology does not require the conventional propagation and vegetative stages and provides an unprecedented level of efficiency and consistency to the Cannabis industry.
" The repeated use of the same Cannabis cell reservoir for two years is an unprecedented scientific achievement in the Cannabis plant kingdom ," said Dr. Yochi Hagay , BioHarvest CTO. " Our ability to keep our Cannabis cells in this "flowering" stage for the past two years versus conventional periods of nine weeks demonstrates the unique efficiency of our technology and puts us on track to reach commercial production using Cannabis-based compounds ."
Ilan Sobel , the CEO, said: " The Company is on track to commercialize its Cannabis-based products in H1/2022. The abilities and achievements discussed above take us closer to such a time when we can bring the power of the Company's BioFarming technology to the Cannabis market. I am pleased with our progress and shall continue to update BHSC's stakeholders periodically ."
Eitan Popper , Co-Founder and former President of MedReleaf, and Chairman of BioHarvest's Board of Advisors, stated: " The fact that BioHarvest has been able to use the same cell reservoir for two years now, is a testament to the robustness of the Company's technology and the quality of its Standard Operating Procedures. Reducing the growth cycle and total production time from no less than the conventional 16 weeks to 3-4 weeks, has immense economic and environmental implications. "
The Company invites all interested investors and media to join the July 8 Shareholder Update Webinar, at 2pm EST . Register Now!
https://app.livestorm.co/st-financial/bioharvest-sciences-q2-shareholder-update-july-8-2021?type=detailed
About BioHarvest Sciences Inc.
Based in Vancouver BC, BioHarvest Sciences Inc. is the developer and exclusive owner of the proprietary and patent-protected BioFarming technology. It is the first and only industrial-scale plant cell technology capable of producing the active plant ingredients without the necessity to grow the plant itself. The Company's technology is non-GMO and has already been validated by VINIA®, the red grapes cells functional food/dietary supplement produced and sold by BioHarvest Sciences Inc. The Company plans to generate significant revenue within the global nutraceutical ingredients and dietary supplements market with VINIA® and other Super Fruit Nutraceutical products. Further, by adapting this technology to the cannabis plant, and building adequate production capacity, BioHarvest Sciences Inc.'s objective is to also become a leading supplier of cannabis for both medicinal and legal recreational purposes. For more information visit: www.bioharvest.com .
BioHarvest Sciences Inc.
Ilan Sobel , Chief Executive Officer
For further information, please contact:
Dave Ryan , VP Investor Relations & Director
Phone: 1 (604) 622-1186
Email: dave@bioharvest.com
Media Contact
Will Hummel
+31639177280
William.Hummel@BOLDTpartners.com
Forward-Looking Statements
Information set forth in this news release includes might include forward-looking statements that are based on management's current estimates, beliefs, intentions, and expectations, and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. In particular, there is no assurance we will be able to commercialize our first Cannabis products in the first half of 2022. Delays and cost overruns may result in delays achieving our objectives obtaining market acceptance and regulatory approvals for geographic expansion is subject to risk and cannot be guaranteed. The success of the Company in demonstrating its ability to consistently grow in solution trichomes from multiple plant strains is not an assurance that the Company will be able to commence commercial production when anticipated or at all. While the company is in the process of constructing a two ton production facility the Company 's current licensing only permits scientific research. Projected sales of Cannabis will require the company to obtain production and / or export licensing which cannot be assured.
All forward-looking statements are inherently uncertain and actual results may be affected by a number of material factors beyond our control. Readers should not place undue reliance on forward-looking statements. BHSC does not intend to update forward-looking statement disclosures other than through our regular management discussion and analysis disclosures.
Neither the Canadian Securities Exchange nor its Regulation Services Provider accept responsibility for the adequacy or accuracy of this release.
Photo - https://mma.prnewswire.com/media/1558311/BioHarvest_Sciences_Inc.jpg
Logo - https://mma.prnewswire.com/media/1558310/BioHarvest_Sciences_Logo.jpg
SOURCE BioHarvest Sciences Inc.