
August 07, 2024
Radiopharm Theranostics (ASX:RAD) is a biopharmaceutical company that develops radiopharmaceutical products for diagnostic and therapeutic applications. With four licensed platform technologies – nanobody, peptide, small molecules and monoclonal antibodies (mAb), Radiopharm aims to commercialize its pipeline for possible licensing and distribution agreements seeking to develop for the diagnosis and treatment of certain cancers.
Radiopharm Theranostics represents a promising investment opportunity in the rapidly growing field of radiopharmaceuticals, leveraging its innovative technology platform and diverse clinical pipeline.
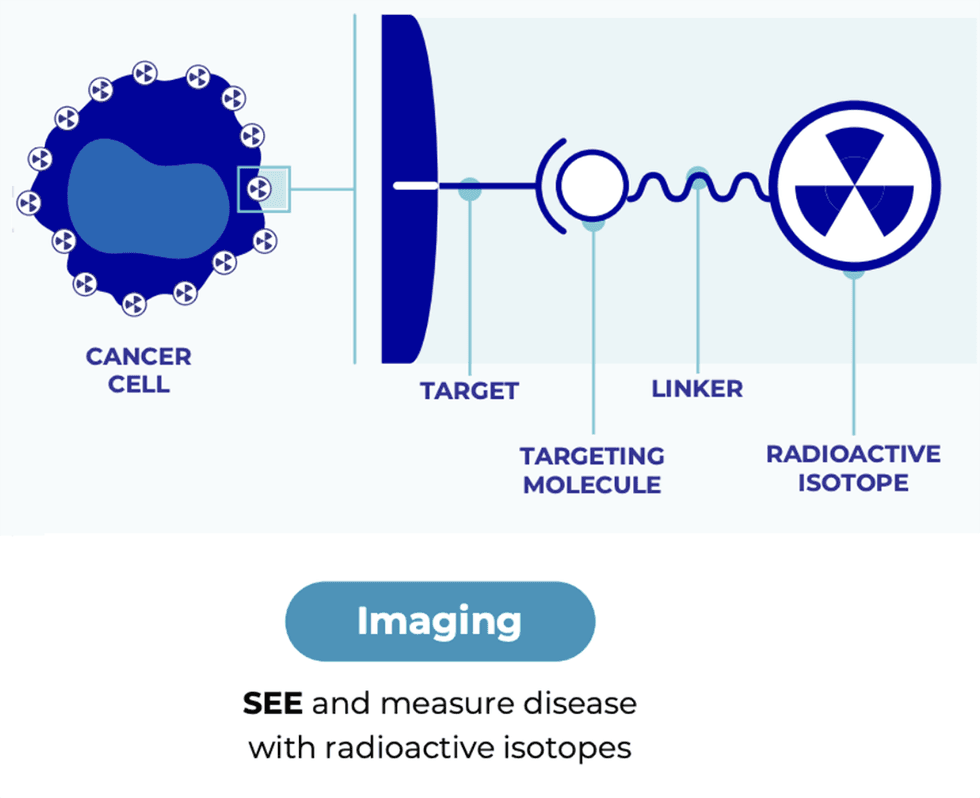
Radiopharm’s clinical stage development in the pipeline include:
- PD-L1 (non-small cell lung cancer indication) - currently in phase 1 in Australia;
- HER2 (breast/gastric cancer indication) - will begin phase 1 trials this year;
- Integrin VB6 (pancreatic cancer indication) - now in Phase I imaging in pancreatic cancer.
- Fatty Acid Synthase (brain METS indication) - preclinical has been completed and with IND approval for Phase IIb Imaging
Company Highlights
- Radiopharm Theranostics is focused on developing and commercializing radiopharmaceutical products and nuclear medicines for both therapeutic and diagnostic applications in precision oncology.
- Radiopharm has four licensed platform technologies – nanobody, peptide, small molecules and monoclonal antibodies (mAb) – with diagnostic and therapeutic applications in both pre-clinical and clinical stages of development.
- The company has received clearance from the US Food and Drug Administration for an investigational new drug application with two INDs (one for RAD 301 and one for RAD 101). Phase 1 for RAD 301 and for RAD 204 is in progress.
- The company aims to commercialize its pipeline for possible licensing and distribution agreements and has secured four platform technologies, which it is seeking to develop for the diagnosis and treatment of certain cancers.
This Radiopharm Theranostics profile is part of a paid investor education campaign.*
Click here to connect with Radiopharm Theranostics (ASX:RAD) to receive an Investor Presentation
RAD:AU
The Conversation (0)
29 August 2025
Appendix 4E and Preliminary Final Report
Radiopharm Theranostics (RAD:AU) has announced Appendix 4E and Preliminary Final ReportDownload the PDF here. Keep Reading...
29 July 2025
Quarterly Activities/Appendix 4C Cash Flow Report
Radiopharm Theranostics (RAD:AU) has announced Quarterly Activities/Appendix 4C Cash Flow ReportDownload the PDF here. Keep Reading...
27 July 2025
RAD receives IND approval from US FDA for Betabart (RV-01)
Radiopharm Theranostics (RAD:AU) has announced RAD receives IND approval from US FDA for Betabart (RV-01)Download the PDF here. Keep Reading...
10 June 2025
RAD Granted US FDA Fast Track for RAD101 Metastases Imaging
Radiopharm Theranostics (RAD:AU) has announced RAD Granted US FDA Fast Track for RAD101 Metastases ImagingDownload the PDF here. Keep Reading...
03 June 2025
RAD Doses 1st Patient in Therapeutic Trial of 177Lu-RAD202
Radiopharm Theranostics (RAD:AU) has announced RAD Doses 1st Patient in Therapeutic Trial of 177Lu-RAD202Download the PDF here. Keep Reading...
Latest News
Interactive Chart
Latest Press Releases
Related News
TOP STOCKS
American Battery4.030.24
Aion Therapeutic0.10-0.01
Cybin Corp2.140.00
