
August 12, 2024
Tryptamine Therapeutics Limited (‘Tryp’ or the ‘Company’) (ASX: TYP), a clinical-stage biotechnology company is pleased to advise it has received highly encouraging, positive results from its recently completed Phase 2a clinical trial conducted in collaboration with the University of Michigan (‘UOM’) (refer ASX announcement: 10 July 2024). The results are both significant and clinically meaningful, and were presented by UOM researchers at the International Association for the Study of Pain (‘IASP’) 2024 World Congress in the Netherlands on 9 August 2024.
- Phase 2a fibromyalgia trial undertaken with University of Michigan (“UOM’) with results presented at the IASP 2024 World Congress on Pain 9 August 2024 in the Netherlands
- All patients dosed with TRP-8802 (TYP’s oral psilocybin formulation), and administered psychotherapy reported an improvement in fibromyalgia pain severity, sleep, pain interference and at least 3 other endpoints measured one month after dosing
- Fibromyalgia is a condition associated with widespread pain – 1m people in Australiai and ~10m people in the USii suffer from it and there are currently limited treatment options
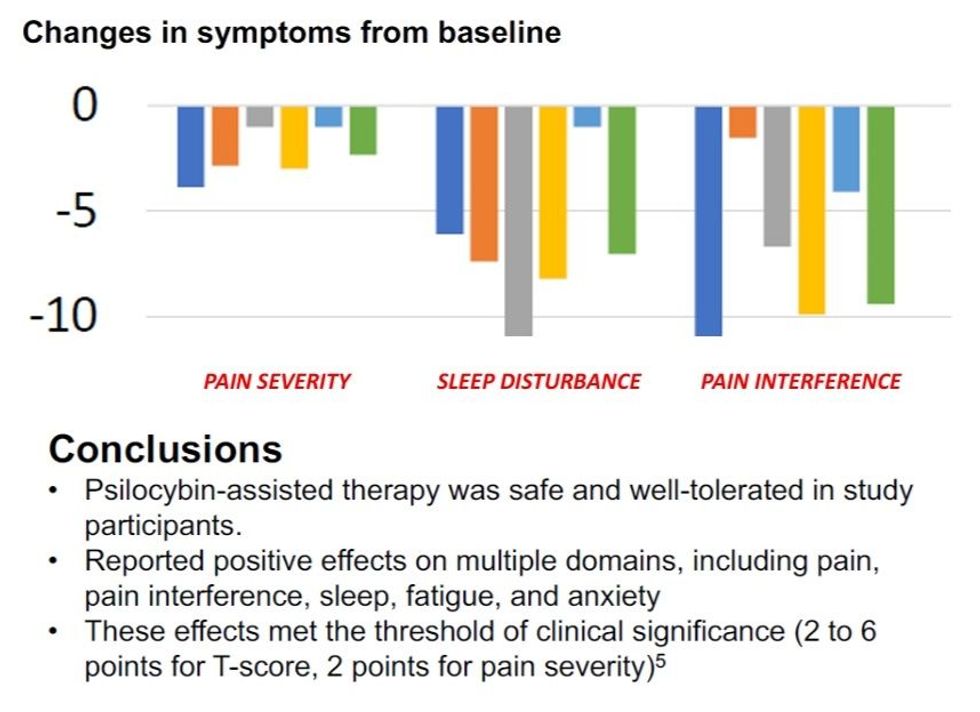
- Results highlighted that there was a clinically meaningful reduction in pain, pain interference and fatigue
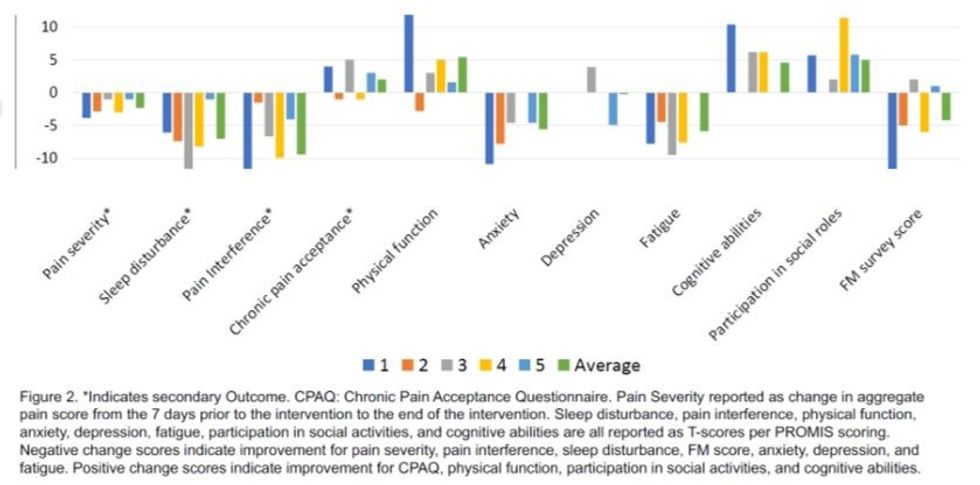
- Patients also reported a number of other improvements including clinically meaningful improvements in quality-of-life measures such as sleep, physical activity, and the ability to participate in daily social activities
- Clinically meaningful reduction in anxiety and improved cognitive abilities were also reported in 4 out of 5 patients dosed
- In addition, one patient reported during follow-up that their sense of smell had returned following a COVID- 19 diagnosis in 2021
- Results highlight the significant potential for psychedelic-assisted therapy as a compelling treatment pathway for fibromyalgia when compared to the inadequacies of incumbent treatments
- Results considerably strengthen Tryp’s IP position and lay a strong foundation for a future trial using TRP- 8803 (IV-infused psilocin) – Phase 2 trial planning now underway and expected to commence H1 2025
- Additional clinical trials progressing well at MGH for patients with IBS, and the beginning of Cohort 3 in the TRP-8803 Healthy Volunteer Study expected to begin soon – updates to be provided in due course
The trial was designed to evaluate TRP-8802 (oral psilocybin) in conjunction with psychotherapy in patients with fibromyalgia, a condition associated with widespread pain and comorbidities that significantly impact daily living and patient well-being. The trial was undertaken by the UOM, a top-ranked public university in the US in collaboration with Tryp.
Results highlighted that 100% of patients experienced reductions in fibromyalgia pain, sleep disturbance and pain interference (refer figures one and two below).
Whilst appreciating the limitations of the small number of patients and the open label nature of the ‘signal’ style study, the results are highly encouraging and considerably strengthen TYP’s intellectual property position. Further, the results validate Tryp’s clinical trial approach targeting nociplastic pain with an initial focus on fibromyalgia and will inform an additional clinical study utilising TRP-8803 (IV-infused psilocin), which is expected to commence H1 2025.
Trial background:
The study was an open-label clinical trial with psychotherapy, seeking to evaluate TRP-8802 (oral psilocybin) in conjunction with psychotherapy in patients with fibromyalgia. A total of five patients were recruited and were administered two doses of TRP-8802 in 15mg initial dose and 25mg second dose formats, two weeks apart. Patients were administered psychotherapy in concert with TRP-8802 and results were compared to baseline values one month following the completion of the second dose.
Click here for the full ASX Release
This article includes content from Tryptamine Therapeutics Limited, licensed for the purpose of publishing on Investing News Australia. This article does not constitute financial product advice. It is your responsibility to perform proper due diligence before acting upon any information provided here. Please refer to our full disclaimer here.
The Conversation (0)
Latest News
Interactive Chart
Latest Press Releases
Related News
TOP STOCKS
American Battery4.030.24
Aion Therapeutic0.10-0.01
Cybin Corp2.140.00