
- Program will integrate existing neurocare clinics with HOPE Therapeutics clinics and will engage the already-installed base of 400+ Apollo® Transcranial Magnetic Stimulation (TMS) machines nationwide.
- Program will unite common treatment protocols and clinical training with neurocare expertise in neuromodulation technology and NRx Pharmaceuticals neuroplastic drug development program.
- Pilot program with a state first-responder agency has demonstrated high rates of success in combining TMS, neuroplastic drug treatment, hyperbaric oxygen, and psychotherapy to achieve remission from PTSD and Depression in First Responders.
- neurocare and NRx look forward to offering an integrated treatment program to patients and payers within driving distance of any American home by year-end 2026.
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP), a clinical-stage biopharmaceutical company and neurocare Group AG today ("The Partners") announced a partnership to create a nationwide network of clinics to offer integrated neuroplastic care for the treatment of Depression, PTSD, and other serious mental health disorders. Pilot programs have shown that combining Transcranial Magnetic Stimulation (TMS) with ketamine and other neuroplastic drugs, along with hyperbaric oxygen therapy and supportive psychotherapy has resulted in a high rate of remission among First Responders with PTSD and Depression.
Recent scientific publications have demonstrated that integration of TMS with neuroplastic drugs has achieved unprecedented response of 87% and remission of 72% in patients with treatment-resistant depression. While these results are early and will require further study, they are highly encouraging. The Partners are additionally exploring joint regulatory paths including clinical trials to address the treatment of Bipolar Depression, Autism, and Traumatic Brain Injury with TMS and neuroplastic drugs, such as NRX-101.
Although there have been a number of clinic chains founded to combine some of these therapies in the past, a fully-integrated nationwide program that combines these neuroplastic modalities with common training protocols, advanced use of Telepsychiatry and Information Technology, and payer outreach has not been achieved. This approach was successful in creating reliable and effective approaches to the delivery of care for renal failure, as demonstrated by Fresenius Medical Care and Davita in past years. neurocare, founded by former Fresenius leaders, has more than a decade of experience in the development of a platform that includes neuromodulation technology (eg rTMS, neurofeedback protocols, qEEG and sleep analysis devices) as well as an online academy and a process software called neurcareOS to guide intake and personalizing of mental health patients. Meanwhile NRx has a decade of experience in developing neuroplastic drugs. In recent years, both organizations have recognized the need for an integrated clinical solution that could be offered to payers on an Accountable Care basis and the need for an Academy program to train a new generation of neuroplastic therapy practitioners.
The partnership will leverage the rapidly-growing base of clinics already owned and founded by neurocare and HOPE Therapeutics (currently 20 in the US), together with offering participation to the currently installed base of 400+ Apollo® TMS devices nationwide. By doing so, the partners aim to offer innovative neuroplastic therapy within driving distance of any home in the United States by the end of 2026. Similarly, the partners aim to offer patients and payers a single point of contact for these challenging CNS diseases that afflict more than 50 million in the US and 500 million worldwide.
Tom Mechtersheimer, neurocare's Founder & CEO said: "We are excited to unite our efforts on a national scale for the benefit of patients and their families. NRx's mission to offer integrated care in their HOPE clinics is fully aligned with the thinking behind our best practice empowering platform of tools and processes. We believe that the pipeline of NRx and our platform complement one another as we seek to offer patients and payers a fully integrated solution that maximizes remission from severe depression and PTSD today and additional debilitating CNS conditions in the future. Our initial focus on First Responders, Active Duty Military, and Veterans is a recognition of the urgent need for effective, integrated treatments."
"For too long, the approach to neuroplastic therapy for depression and PTSD has been highly fragmented, with some clinics offering ketamine, some offering TMS, some offering hyperbaric therapy but almost no fully integrated solutions that also provide ongoing psychotherapy and patient monitoring," said Dr. Jonathan Javitt, Founder, Chairman, and CEO of NRx Pharmaceuticals and HOPE Therapeutics. "More importantly, in our partnership with neurocare and their rapidly-growing base of more than 400 TMS devices across the US, we seek to be able to treat any American within driving distance of home by the end of 2026 and to offer a single point of accountable care to payers. Just as our HOPE Therapeutics clinics already have contracts with the VA Community Cares Program and the Department of War TRICARE program, we aim to engage commercial and governmental payers to offer a fully integrated treatment program to patients at the highest need in what is today a highly fragmented market."
Dr. Javitt and Mr. Mechtersheimer will be meeting jointly with investors and others at the upcoming JP Morgan Healthcare Conference in San Francisco.
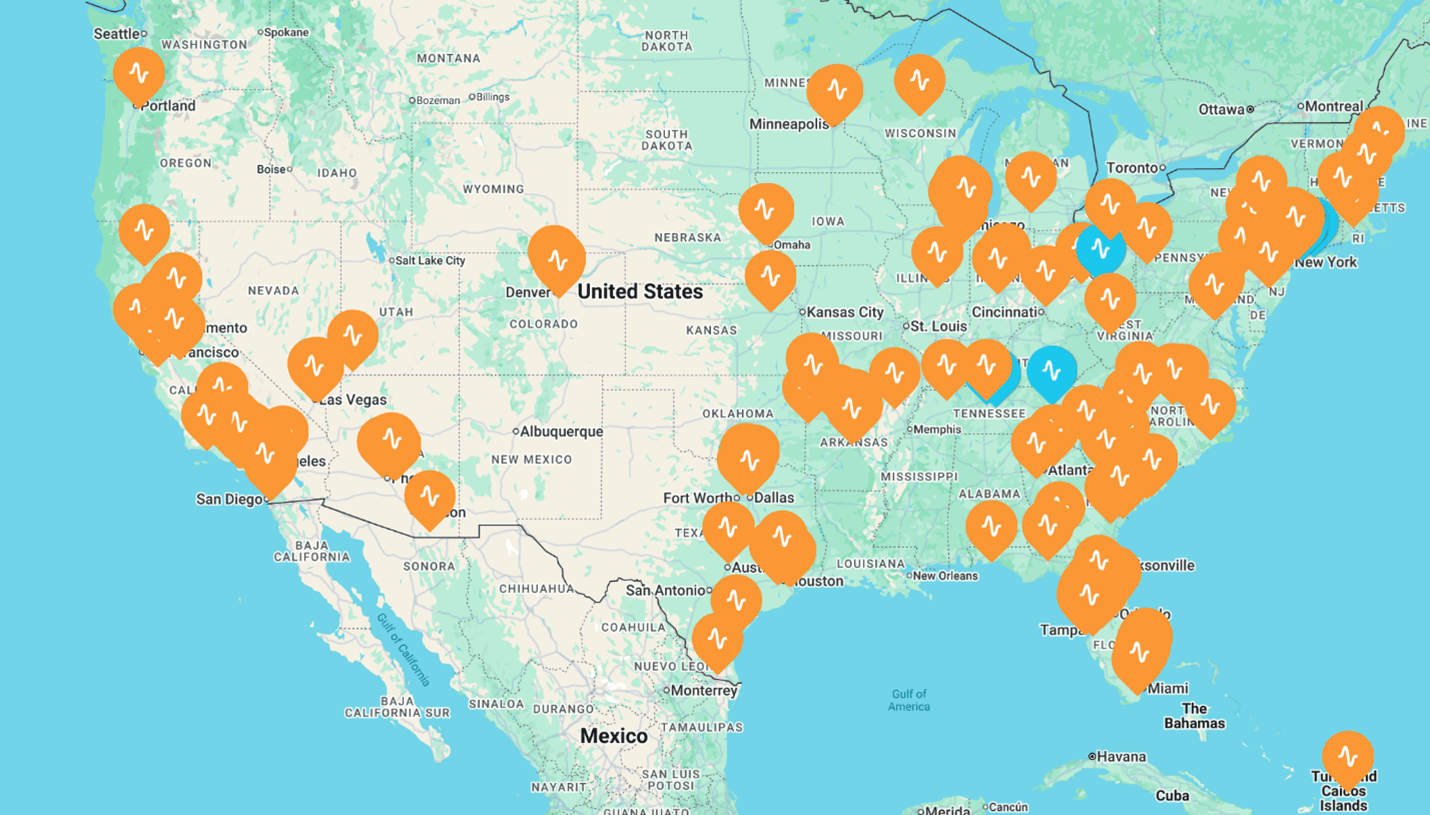
Current neurocare, HOPE, and Apollo US locations (source: neurocare group AG and HOPE Therapeutics, Inc.)
About neurocare group AG
neurocare group AG (MUNICH, Germany and Atlanta, GA) has developed a best practice platform for neuroplastic therapy targeting mental health conditions. The platform is led by its top selling Apollo® Transcranial Magnetic Stimulation (TMS) device with more than 400 installations in the US alone to date. neurocare empowers clinicians to offer their patients precise TMS therapy through EEG-guidance complemented by neurofeedback and sleep hygiene devices. neurocare's platform also includes neurocare OS, a built-in patient and data management system as well as the neurocare Academy, an online/in-person medical education platform that trains and certifies physicians, therapists, and technologists in TMS and other neuroplastic therapies. With the platform's technologies and process tools, neurocare empowers clinicians in developing personalized patient journey's including a detailed intake first. The outcomes rapidly reduce depression, suicidality, and other symptoms exceeding the results previously shown with antidepressants or CBT alone.
neurocare was founded and is led by industry veterans and TMS pioneers who developed and launched the first commercially-successful TMS technologies and continue to advance the technology envelope. Beyond its 400+ customer sites in the US, neurocare operates 40 clinics across the US, the UK, the Netherlands and Australia and is selling its platform tools and technologies internationally. Since inception in 2015 neurocare has grown to sales of annual sales exceeding EU35 million in 2025 and expects to exceed EU50 million in 2026.
About NRx Pharmaceuticals, Inc.
NRx Pharmaceuticals, Inc. (www.nrxpharma.com), is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal depression, chronic pain, and PTSD. The Company is developing NRX-100 (preservative-free intravenous ketamine), under and ANDA which has been filed with the US Food and Drug Administration with an expected decision date of July 2026, and has been awarded Fast Track Designation for the treatment of Suicidal ideation in Depression, including Bipolar Depression for filing as an innovative drug. NRX-101, (oral D-cycloserine/lurasidone) has been awarded Breakthrough Therapy Designation for the treatment of suicidal bipolar depression.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com), a subsidiary of NRx Pharmaceuticals, is a Healthcare delivery company that is building a best-in-class network of interventional psychiatry clinics to offer ketamine and other neuroplastic medications, transcranial magnetics stimulation (TMS), Hyperbaric Oxygen Therapy, and other lifesaving therapies to patients with suicidal depression and related disorders, together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. The Company has reported regulatory milestones as they have been achieved but has not predicted the outcome of any future regulatory determination. You should not
place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, including uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy, and, among other things, liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, the Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
For further information:
Brian Korb
Managing Partner, astr partners
(917) 653-5122
brian.korb@astrpartners.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/0e221354-b2d7-4aa6-b832-2170c6f657c4
