August 30, 2023
Hydration solutions company The Hydration Pharmaceuticals Company Limited (ASX: HPC) (“Hydralyte North America” or “the Company”) is pleased to provide its half year activities report and Appendix 4D for the six month period ended 30 June 2023 (the “half year” or “1H FY2023”).
KEY HIGHLIGHTS
- Record net revenue achieved for the half of US$5.3m, representing 28% growth on prior corresponding period ('PCP') (1H FY2022: US$4.1m)
- Strong half year revenue result underpinned by monthly net sales of US$1.09m in June - demonstrating strong traction ahead of North American summer months
- Loss from ordinary activities improved 17% following ongoing review of cost base and implementation of initiatives to reduce capital expenditure
- Gross margin remains stable at 49% or US$2.6m, representing a 1% decrease from the last half (2H FY2022: 49%)
- Continued execution of strategy to decrease expenditure and extend cash reserves through significant reduction in marketing costs following heavy brand investments in FY2022
- 6 new product launches well progressed – One new product shipped in Q2 and more launching in Q3 to underpin ongoing sales growth
Financial overview:
Revenue for the half year increased 28% on PCP (1H FY2022: US$4.1m) and 7% on the previous half (2H FY2022: US$5.0m) resulting in record of US$5.3m. The rise in revenues is attributed to higher shipments into new and existing North American retailers driven by record retail shelf sell-through, new product launches and ongoing sales to retail customers through eCommerce channels.
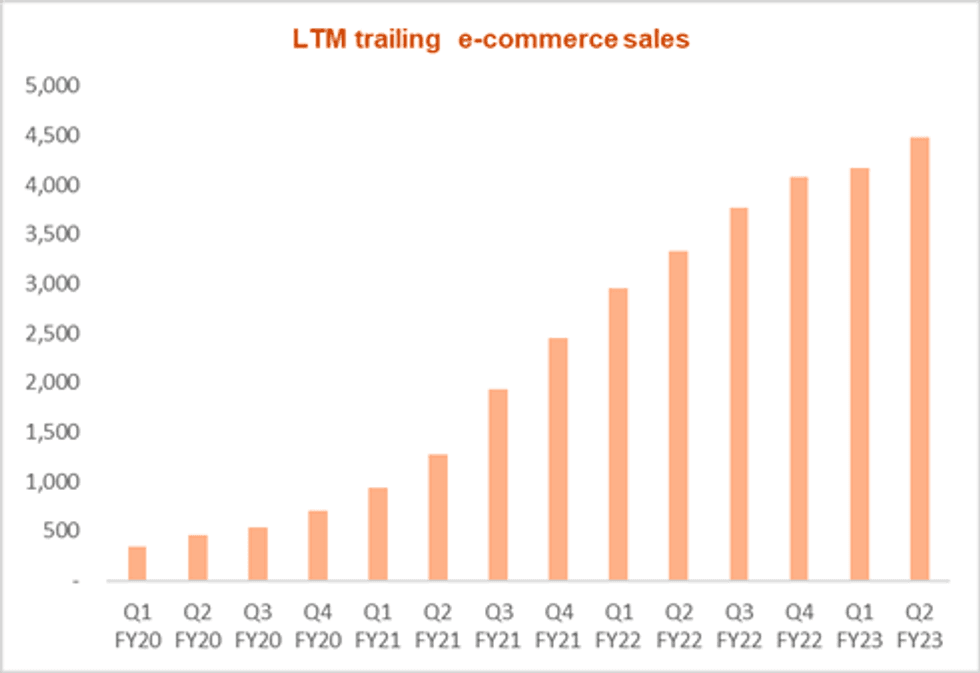
The following chart displays the eCommerce trailing 4-quarter growth through Q2 FY2023:
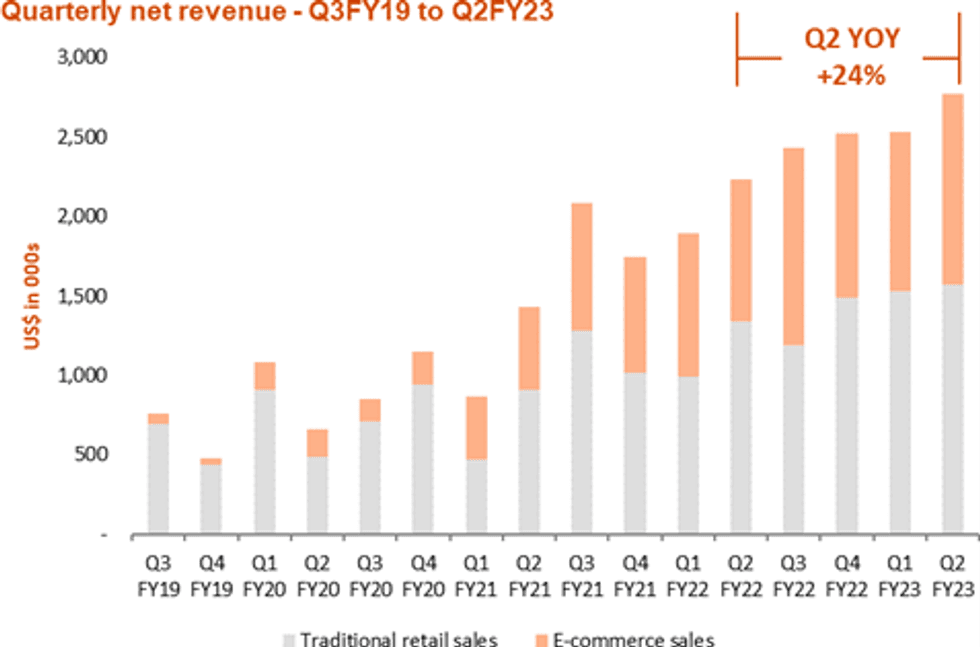
The following chart displays the strong YoY growth (Q2 FY2023 beat seasonally highest Q3 FY2022):
Alongside a material increase in revenue, the Company considerably reduced sales and marketing spend for the half to US$3.7m, down from US$5.3m in 2H FY2022, while sales and marketing as a percentage of net sales decreased from 107% in 2H FY22 to 70% in 1H FY23. Digital Advertising spend was reduced to US$1.0m down from US$1.7m in 2H FY2022.
Click here for the full ASX Release
This article includes content from The Hydration Pharmaceuticals Company Limited, licensed for the purpose of publishing on Investing News Australia. This article does not constitute financial product advice. It is your responsibility to perform proper due diligence before acting upon any information provided here. Please refer to our full disclaimer here.
The Conversation (0)
30 May 2024
Hydralyte International
Leveraging a Rapidly Expanding Hydration Market in North America
Leveraging a Rapidly Expanding Hydration Market in North America Keep Reading...
Latest News
Interactive Chart
Latest Press Releases
Related News
TOP STOCKS
American Battery4.030.24
Aion Therapeutic0.10-0.01
Cybin Corp2.140.00