Investor Insight
With a strong IP portfolio, deep clinical-stage pipeline, global collaborations and an experienced leadership team, Invion is poised for transformative value creation as it enters the next phase of clinical and commercial growth.
Overview
Invion Limited (ASX:IVX) is a clinical-stage Australian life sciences company focused on transforming photodynamic therapy (PDT) into a next-generation solution for treating cancer and infectious diseases. The company’s core value lies not just in preclinical promise, but in real-world clinical validation.

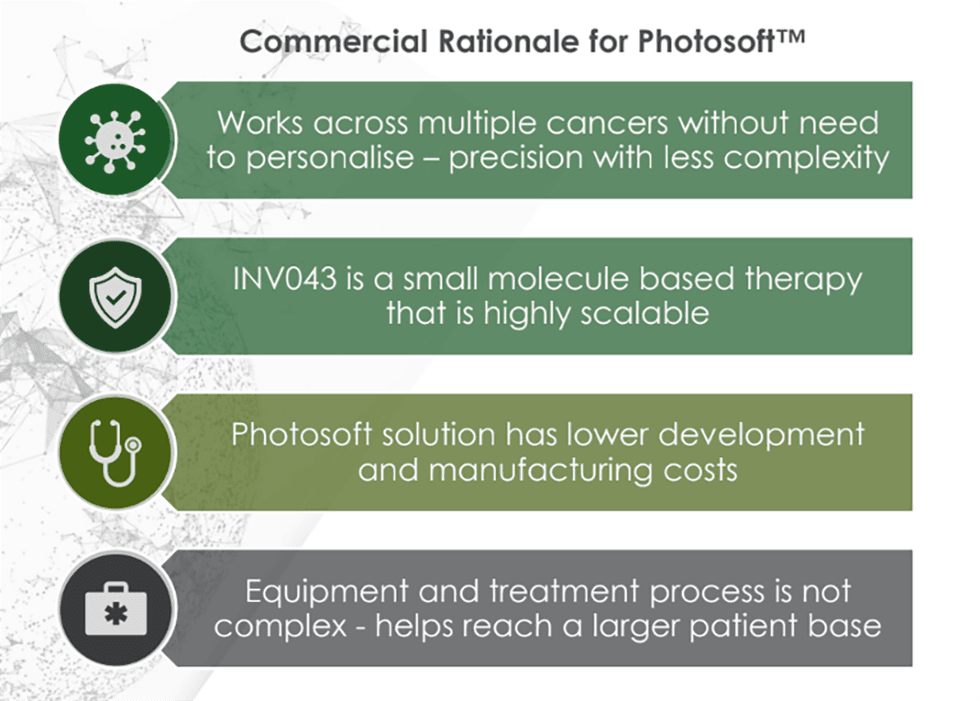
At the heart of Invion’s platform is Photosoft, a proprietary suite of next-generation photosensitizers that selectively target diseased cells and, upon light activation, induce a precise oxidative stress response to destroy them. Unlike conventional PDTs that suffer from toxicity and off-target effects, Photosoft compounds are engineered for enhanced safety, specificity, immune activation, and theragnostic utility. INV043, Invion’s lead cancer drug candidate, has demonstrated up to 80 percent tumor control in combination with immune checkpoint inhibitors, and is now in human trials.The company is actively progressing a broad pipeline across multiple indications, including:
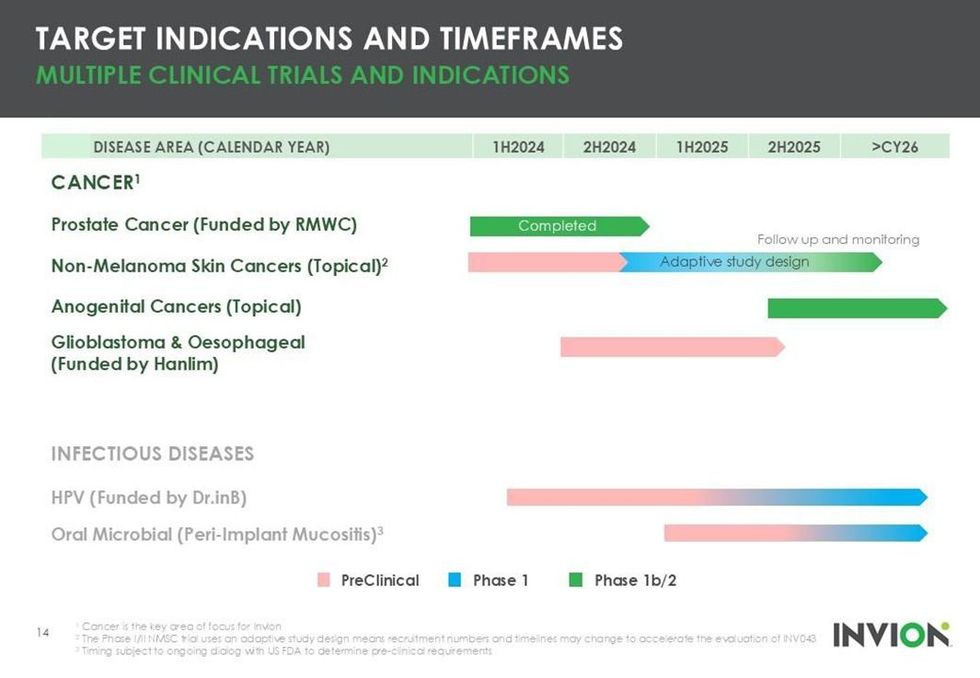
- A Phase I/II trial in non-melanoma skin cancer (NMSC), now dosing in Australia
- An anogenital cancer trial in collaboration with Peter MacCallum Cancer Centre, scheduled to begin in 2025
- A completed investigator-led Phase II prostate cancer trial, showing a 40 to 44 percent response rate with a strong safety profile
These trials signal a critical shift for Invion from preclinical research to meaningful clinical validation, spanning both systemic and topical formulations. The safety data from the NMSC trial is expected to support accelerated development of the anogenital cancer program, given both use the same topical compound.
The clinical results so far have been consistent with the findings from the preclinical studies – showing INV043 to be very safe with promising efficacy signals. The next set of trials aim to further solidify the potential for the technology, where the one drug can treat multiple cancers, improve patient outcomes when using checkpoint inhibitors in combination with INV043 and demonstrate its diagnostic and theragnostic potential.
Beyond internally funded trials, Invion is strategically expanding its clinical and commercial footprint through non-dilutive, global partnerships. In South Korea, Hanlim Pharm is funding the preclinical development of Photosoft for glioblastoma multiforme — a highly aggressive brain cancer — and oesophageal cancer, both of which present major unmet needs. These programs are fully financed by Hanlim, with Invion retaining all intellectual property

Similarly, Dr. I&B Co. (Dr.inB), another South Korean group, is backing the development of Photosoft for human papillomavirus (HPV) in a new proof-of-concept trial. This collaboration not only funds a novel therapeutic area outside of oncology but underscores the versatility of Photosoft as a multi-indication, platform technology. Invion bears none of the clinical trial costs and maintains all rights to future commercialization.

In parallel, Invion is expanding the Photosoft platform into infectious diseases, where preclinical studies have demonstrated broad-spectrum antimicrobial activity. The compound has proven effective in vitro against:
- Antibiotic-resistant “superbugs”
- Fungal and bacterial infections
- SARS-CoV-2 (Omicron)
- Oral and periodontal conditions such as peri-implant mucositis
These results reflect Invion’s long-term vision of developing a scalable, accessible and affordable therapy platform that addresses both high-burden cancers and the growing global threat of antimicrobial resistance. With Photosoft, the company is building a foundation not just for treating disease — but for reshaping therapeutic accessibility and patient outcomes worldwide.
Company Highlights
- Clinical-stage Pipeline in Multiple Indications: Successfully completed Phase II prostate cancer trial, ongoing Phase I/II skin cancer trial, and anogenital cancer trial initiating in 2025. Multiple cancer and infectious disease programs underway.
- Photosoft Platform Technology: Combines cancer selectivity, immune system activation, and minimal toxicity. Preclinical studies show INV043 can regress multiple cancers, deliver superior safety and efficacy and improve tumour control to 80 percent in combination therapy studies with blockbuster ICIs (vs 12 percent with ICIs alone).
- Renowned Partners & Global Pharma-funded Collaborations: Working with distinguished research institutions like Peter MacCallum Cancer Centre and Hudson Institute of Medical Research. Further, Hanlim Pharm (GBM, oesophageal cancer) and Dr.inB (HPV) are funding multiple programs without requiring Invion to contribute capital or give up IP.
- Theragnostic Capability: Photosoft compounds enable both treatment and imaging, allowing for highly precise cancer targeting and enhanced surgical decision-making.
- Strong Clinical and IP Foundation: GMP-grade INV043 manufactured and patented in Australia, with global IP protection extending to at least 2041.
- Compelling Upside: Following a share consolidation and reduced overhangs, IVX offers significant re-rating potential with multiple clinical readouts expected over the next six to 12 months.
Key Programs
Non-melanoma Skin Cancer

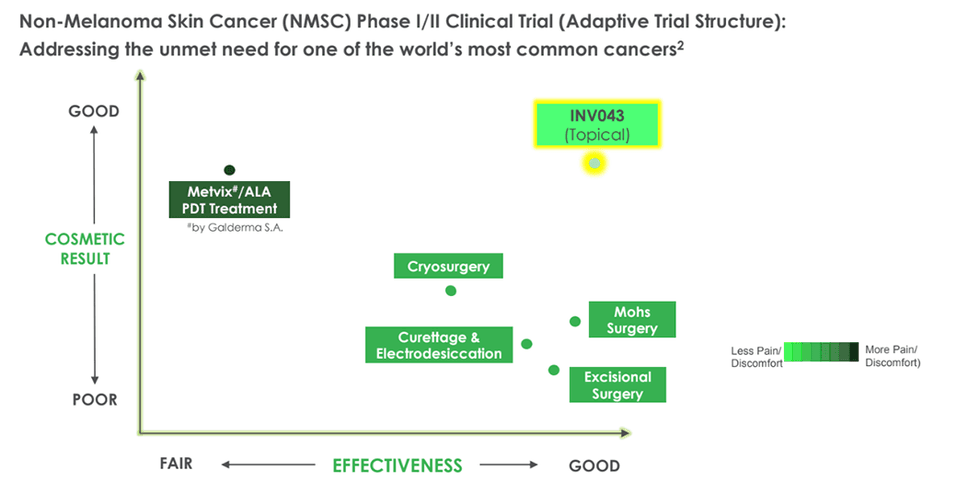
Invion’s leading active trial is a Phase I/II adaptive clinical study targeting non-melanoma skin cancer — a condition that represents 98 percent of all skin cancers and constitutes a substantial public health burden in Australia. Using a topical formulation of INV043, the trial is designed with a 3+3 structure allowing real-time protocol optimization for safety and efficacy. The formulation offers significant cosmetic and pain-reduction benefits over existing approved PDT treatments like Metvix (by Galderma S.A.) and excisional surgery, both of which have limitations related to scarring and pain. Dosing of the first patient commenced in December 2024, with ongoing recruitment and interim data expected in the second half 2025 or early 2026. The skin cancer program not only represents a potentially fast path to market but also supports downstream programs, such as the anogenital cancer study, through shared formulation and safety data.
Anogenital Cancers (Peter Mac Collaboration)
The anogenital cancer program is a major upcoming milestone for Invion, developed in collaboration with the prestigious Peter MacCallum Cancer Centre in Melbourne, Australia, one of the leading oncology research centres in the world. Utilizing the same topical INV043 formulation as the non-melanoma skin cancer trial, this Phase I/II study will leverage safety data from the trial to accelerate approval timelines. The anogenital trial targets high-risk lesions and cancers with limited therapeutic options, and benefits from the scientific and operational expertise of Peter Mac. Preclinical data showed exceptional synergy when INV043 was used in combination with immune checkpoint inhibitors (anti-PD-1), achieving up to 80 percent tumor-free responses in models of anal squamous cell carcinoma, compared with a circa 12 percent response rate with aniti-PD-1 alone. This program exemplifies Invion’s theragnostic strength, where the compound also enables fluorescent visualization of tumor margins to aid surgical decision-making.
Prostate Cancer (Phase II Completed)
INV043 has completed a Phase II investigator-led clinical trial in prostate cancer, funded by the RMW Cho Group. The trial involved sublingual systemic administration of the compound followed by targeted light therapy. Following COVID-related disruptions, a second cohort of 16 patients was successfully treated and evaluated using PSMA-PET scans and RECIST criteria. Results were compelling: 44 percent of patients showed no detectable cancer via PSMA-PET scan three months post-treatment, while 40 percent demonstrated partial or stable response by MRI. The therapy was extremely well-tolerated, with no serious adverse events and only mild treatment-emergent side effects. These findings serve as clinical proof-of-concept for systemic delivery of INV043, highlighting its potential for deeper-seated cancers and reinforcing its safety and scalability.
Glioblastoma and Oesophageal Cancer (Hanlim Pharma Collaboration)
Through a strategic partnership with South Korean pharmaceutical company Hanlim Pharma, Invion is advancing preclinical programs for two highly aggressive and deadly cancers: glioblastoma multiforme and oesophageal cancer. Hanlim is funding both programs entirely, allowing Invion to retain all IP and avoid capital outlay. These programs are exploring INV043’s efficacy in some of the most treatment-resistant solid tumors, with early-stage studies underway and updates expected in 2025. If successful, this collaboration could lead to regional licensing or joint ventures in Asia, significantly expanding Invion’s global footprint.
HPV and Infectious Diseases (Dr.inB Collaboration)
Dr.inB, a leading PDT innovator in South Korea, is partnering with Invion to develop Photosoft-based treatments for HPV-related conditions, including genital warts and potentially HPV-linked cancers. The program, which includes proof-of-concept human trials, is entirely funded by Dr.inB, and Invion retains all IP and commercialization rights. Beyond HPV, Photosoft has demonstrated broad-spectrum antimicrobial potential against antibiotic-resistant bacteria, fungi and viruses, including SARS-CoV-2. This opens the door to applications in periodontal disease, peri-implant mucositis, and other infectious conditions. PDT’s unique mechanism of action — using light to generate oxidative stress — renders it immune to resistance development, making it an ideal candidate for combatting antimicrobial resistance, one of the top 10 threats to humanity identified by the World Health Organization.
Management Team
Thian Chew – Executive Chairman & CEO
Thian Chew brings over two decades of executive and advisory experience in healthcare and finance. He is the co-founder of Chronic Airway Therapeutics and a board advisor at Stanford Medicine’s Center for Asian Health Research and Education (CARE). Formerly an executive director at Goldman Sachs and director at KPMG Consulting, Chew combines strategic vision with operational rigor. He is an adjunct professor at the University College London and associate professor at HKUST, holding dual an MBA from Wharton School (Palmer Scholar) and an MA from the Lauder Institute, University of Pennsylvania.
Robert Ramsay – Scientific Advisor
A world-renowned expert in immunotherapy and translational cancer biology, Robert Ramsay is a senior scientist at Peter MacCallum Cancer Centre and has over 30 years of oncology research experience. He was instrumental in demonstrating the synergy between INV043 and checkpoint inhibitors, leading to his appointment as a key advisor. Ramsay also served as president of the Australian Society for Medical Research.
Scott Carpenter – Program Director
Scott Carpenter brings cross-functional expertise in regulatory affairs, business development and stakeholder engagement. He previously held leadership roles at Starpharma, AusBiotech and Bayer CropScience and holds an MBA from Melbourne Business School.
Sebastian Marcuccio – Medicinal Chemistry Lead
The co-inventor of Invion’s PDT patents, Sebastian Marcuccio is the founder of Advanced Molecular Technologies and has a deep background in pharmaceutical R&D, including with CSIRO. He is currently adjunct professor at La Trobe University and holds a PhD in organic chemistry.
Kim Steel – Clinical Trial Director
With more than 18 years of experience managing global Phase I-IV drug and device trials across 14 countries, Kim Steel has worked with Novotech and Pacific Clinical Research. She is managing director of SAPRO Consulting, leading operational delivery for Invion’s ongoing trials.
Alexander Bennett – Technical Advisor, Light Devices
A veteran of scientific instrumentation development, Alexander Bennett brings 35+ years of experience designing medical and forensic light systems. He led PDT light source trials at Peter MacCallum Cancer Centre and ensures the clinical precision of INV043’s light activation protocol.