Overview
Cardiex Limited (ASX:CDX) is an ASX-listed medical technology company leveraging its proprietary SphygmoCor® technology to develop and market vascular biomarker technologies and digital solutions focused on the world’s largest health disorders. The company’s groundbreaking technology – SphygmoCor® – set the benchmark for noninvasive measurement of central aortic pressures and related arterial health characteristics, collectively referred to as vascular biomarkers.

While measuring arterial health parameters had always been considered clinically beneficial, it was not considered for routine out-patient clinical use as it previously required an invasive catheterization procedure with a pressure sensor inserted into the aorta. Founded on 40 years of hemodynamics studies and backed by 20+ years of research, Cardiex’s SphygmoCor technology employs non-invasive techniques to assess "central aortic waveforms," offering valuable insights into various clinically significant arterial health parameters including arterial stiffness, central blood pressure (the pressure at the heart), pulse pressure, and crucial indicators of vascular health for major organs such as the heart, brain and kidneys.
Assessing central blood pressure directly at the heart is deemed superior to conventional blood pressure measurements taken at the arm, primarily owing to the heart's proximity to vital organs. Cardiex’s FDA-cleared devices replace traditional blood pressure technology for first-line screening and monitoring of arterial health status. The unique physiologic insights from the company’s devices provide clinically relevant information that helps guide treatment decisions and offer profound benefits for all members of the healthcare community:
For Healthcare Providers: Enable physicians to make more informed treatment decisions based on clinically relevant vascular health data.
For Patients: Give patients the tools to make better decisions about their own health.
For Pharmaceutical Companies: Generate reliable, real-world, clinically relevant data to accelerate drug development and commercialization.
For more than two decades, the company’s SphygmoCor technology has set the benchmark for vascular biomarker assessments, adopted by premier hospitals and pharmaceutical giants worldwide. SphygmoCor is the chosen technology for measuring central blood pressure in all of the "top 20 hospitals" in the US and has played a crucial role in the clinical trials of leading firms including Bayer, AstraZeneca, Roche, Novartis and GlaxoSmithKline.

Between 2002 and 2023, CDX received five FDA clearances, the latest for the CONNEQT Pulse, a first-of-its-kind connected vascular biometrics monitor.
Cardiex devices have previously been sold and used exclusively in clinical settings – principally by specialist clinicians, for research, and by pharmaceutical companies for drug assessment. The introduction of CONNEQT Pulse represents a significant shift for Cardiex, allowing the company to enter the connected care market and transition from niche segments to the mass healthcare market. Priced comparably to a home health monitor, the CONNEQT Pulse is positioned for widespread adoption and can be deployed at scale in general healthcare practices, homes, or any location where patients are present.
The CONNEQT Pulse will also bolster Cardiex's portfolio in clinical trial solutions with the introduction of a decentralized clinical trial (DCT) platform. The Cardiex DCT platform empowers clinical trial managers to remotely monitor thousands of patients in their homes, enabling pharmaceutical companies to more effectively evaluate potential vascular outcomes across various trial phases. This leads to increased efficiency and cost-effectiveness in trial outcomes.

Furthermore, in response to the growing demand for proactive health monitoring, Cardiex will be launching an innovative wrist-worn device that leverages the SphygmoCor technology to deliver a medical grade wearable with capabilities far beyond conventional health trackers. The CONNEQT Band will be a wearable “cuffless” device designed to monitor vascular health in patients as well as to provide general health insights to consumers.
Cardiex’s goal is to establish a holistic ecosystem that promotes cardiovascular well-being and empowers users to proactively manage their health as an integral part of individuals' health routines, contributing to a paradigm shift in preventive cardiovascular care.
The company’s first-mover advantage and exclusive technology FDA-cleared for noninvasive measurement of central pulse pressures and vascular biomarkers across all adult demographics grant it a distinctive market position. CDX recently secured AU$14 million in funding, which is enough to steer the company towards profitability.
With the entry into the connected care home market with the CONNEQT Pulse, CDX is transitioning towards a recurring revenue model based on monthly subscription fees. This should excite investors, given that the recurring revenue model will receive a higher multiple by the market, thereby boosting the company's valuation.
Company Highlights
- Cardiex Limited is an ASX-listed medical technology company that develops and markets vascular biomarker technologies and digital solutions for the world’s largest health disorders.
- The company’s offerings encompass FDA-cleared medical and home health devices alongside digital solutions tailored for managing health and wellness, delivering unique physiologic insights that inform clinical treatment decisions.
- The company’s groundbreaking technology – named SphygmoCor® – set the benchmark for measuring central aortic pressures and related arterial health characteristics, collectively referred to as vascular biomarkers.
- These biomarkers, extensively researched and detailed in numerous peer-reviewed journals, assess cardiovascular risk and guide disease management by predicting organ damage (such as the heart, brain, and kidneys) and outcomes (including heart failure, ischemic heart disease, and stroke), significantly influencing disease prognosis and clinical treatment.
- CDX received five FDA clearances from 2002 to 2023, the most recent for the CONNEQT Pulse, a world-first connected vascular biometric monitor. The CONNEQT Pulse targets new and significant healthcare channels and transitions the company towards a recurring revenue model based on monthly subscription fees.
- CDX's technology is uniquely FDA-cleared for noninvasive measurement of central pulse pressures and vascular biomarkers across all adult demographics – thereby granting it a distinctive competitive market position. With a significant funding round recently secured, CDX is poised for profitability ahead.
Products and Brands
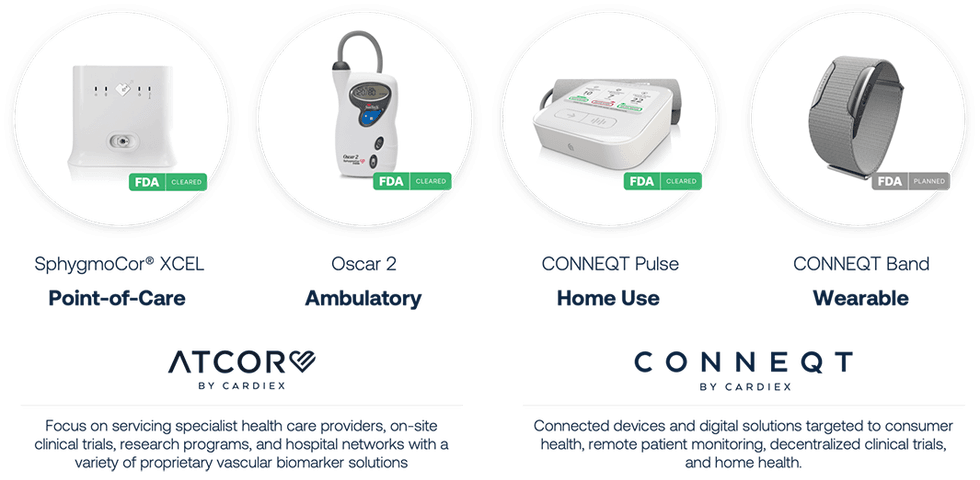
The company’s solution is organised under two brands: ATCOR and CONNEQT.

ATCOR
ATCOR offers solutions for hospitals, research and pharma, and specialist clinician markets. ATCOR's solutions have been utilized in 46 pharmaceutical clinical trials thus far, across more than 1,700 study sites worldwide, and have been highlighted in over 2,400 peer-reviewed studies showcased in top-tier medical journals. It offers two devices – the company’s flagship device, the SphygmoCor XCEL, and the SunTech Oscar 2 with “SphygmoCor Inside”.
SphygmoCor XCEL: The SphygmoCor XCEL measures central and brachial blood pressures, providing insights into cardiovascular function. Additionally, it assesses numerous other clinically relevant vascular biomarkers, offering a comprehensive understanding of arterial stiffness and health. These advanced physiological measurements enable early detection of potential cardiovascular issues, guiding targeted interventions for maintaining or improving overall cardiovascular health.
SunTech Oscar 2 with “SphygmoCor Inside”: Jointly developed and marketed by SunTech Medical and Cardiex, the Oscar 2™ ABPM with “SphygmoCor Inside” produces a suite of unique vascular health features and health insights captured by the SphygmoCor technology over a continuous period. It provides valuable diagnostic information that traditional ambulatory blood pressure monitors are incapable of measuring.
CONNEQT
CONNEQT is focused on devices and solutions for home health, remote patient monitoring, and decentralized clinical trials. Cardiex offers two devices under the CONNEQT brand – the recently FDA-cleared CONNEQT Pulse and the upcoming CONNEQT Band wearable. The company estimates CONNEQT’s total addressable market opportunity to be nearly $283 billion across three markets – remote patient monitoring ($175 billion), health wearables ($104 billion), and decentralized clinical trials ($14 billion).
CONNEQT Pulse: The CONNEQT Pulse provides measurements of both central and brachial blood pressures, alongside vascular biomarkers reflecting arterial stiffness and overall vascular health—metrics once exclusively only available to specialist clinics, research, and pharmaceutical companies. As a Bluetooth-enabled device, it empowers patients and health enthusiasts to track their arterial health from home, employing the same advanced tools used by top cardiologists in research centers and clinics.
Physicians can prescribe the CONNEQT Pulse to patients requiring heart health monitoring. Patient data seamlessly integrates with the CONNEQT Patient Management Portal (CPMP), a HIPAA-compliant cloud-based tool that enables healthcare providers to remotely track patients' arterial health by way of a tablet. Furthermore, consumers and patients can access comprehensive arterial health insights, coaching, lifestyle programs, and additional health resources via the CONNEQT app.
CONNEQT Band: CONNEQT Band is a world's-first dual sensor arterial health wearable device featuring an innovative design with dual (wrist-and finger-based) photoplethysmography (PPG) optical sensors. The wrist-based sensor continuously captures physiological data such as heart rate, respiration, stress, activity, sleep, and pulse oximetry, while our patented on-demand finger-based side sensor uniquely derives clinically meaningful vascular biomarkers representing arterial stiffness and cardiovascular health.
By combining data from both sensors, the CONNEQT Band offers users a comprehensive view of their overall cardiovascular health which has never been available outside of a physician’s office. The CONNEQT Band is also supported by the CONNEQT app, available for both iOS and Android devices, which serves as a hub where users can easily access detailed reports, track their progress over time, explore content, and receive personalized health insights and recommendations.
The CONNEQT Band is currently in the process of FDA submission preparation. This innovative device is poised to extend the company's portfolio in connected health technology, offering users a new, user-friendly tool for monitoring their health metrics. With its cutting-edge features, the CONNEQT Band is expected to make a significant impact in the way individuals manage their wellness, bridging the gap between advanced health monitoring and everyday convenience.
A significant global market opportunity in wearable health devices is driven by nearly 1.3 billion hypertensive and other vascular disease patients. Some of the other wearable device firms have attracted significant funding at high valuations in recent times. For instance, Oura Health, a Finnish company that makes smart rings for tracking sleep and physical activity, was valued at $2.55 billion in April 2022.

Board of Directors & Management
Craig Cooper – Chief Executive Officer and Executive Director
Craig Cooper has established numerous prosperous health, digital media, technology and wellness ventures. Notably, he co-founded Boost Mobile, a prominent telecommunications company recognized as one of the leading mobile phone businesses in the USA. He is acknowledged as a distinguished authority and influential figure on a global scale in mobile and wireless technology, as well as businesses related to digital health and medical technology. His venture capital endeavors have secured over AU$3 billion, financing some of the most impactful global digital media technology companies. Cooper is also a principal of C2 Ventures, Cardiex’s largest shareholder.
Niall Cairns – Executive Chairman
Niall Cairns boasts a successful 25-year record of investing in both private and public companies. He has played a pivotal role in advancing the global expansion of more than 50 enterprises spanning various sectors, including digital media, agtech, medtech, consumer internet, and SaaS-based businesses. Cairns is also a principal of C2 Ventures, Cardiex’s largest shareholder.
King Nelson – Non-executive Director
King Nelson brings over 30 years of extensive experience in medical devices. He was previously the president and CEO at Uptake Medical Corporation, concentrating on treatments for emphysema and lung cancer. Before Uptake, he served as president and CEO of Kerberos Proximal Solutions, a company later acquired by FoxHollow Technologies. He was also president and CEO of VenPro, a heart valve business acquired by Medtronic. King also accumulated 19 years of experience with Baxter International and American Hospital Supply Corporation, progressing through various roles with increasing responsibilities. These roles included serving as division president for Dade Diagnostics, Bentley Labs, and Baxter’s Perfusion Services. Nelson is currently the CEO at Q’Apel Medical, a medical device company specializing in neurovascular disease.
Charlie Taylor – Non-executive Director
Charlie Taylor has over three decades of international advisory experience and recently concluded his tenure as senior partner at McKinsey, where he oversaw the health and public sector practice. Taylor has counseled numerous private and public sector healthcare organizations in Australia and globally, covering areas such as strategy, digitalization, operational enhancements, growth transformations, international expansion, supply chain management, mergers and acquisitions, and board governance. He is a non-executive director of Healius, a leading Australian health diagnostics company, and a part-time senior board advisor at McKinsey for the health and public sector practice.
Sanjeev Bhavnani, M.D. – Chief Clinical Officer
Dr. Sanjeev Bhavnani has served as a senior medical officer at the Digital Health Center of Excellence within the FDA's Center for Devices and Radiological Health (CDRH), overseeing clinical and scientific initiatives concerning digital health and medical devices incorporating artificial intelligence.
Bhavnani is also currently a senior cardiologist and principal investigator of digital health and machine learning at Scripps Clinic in San Diego, California, where he leads programs to develop and validate new technologies and to evaluate the safety and effectiveness of DHTs, nanosensors, cloud-based analytical platforms, handheld imaging technologies, AI/ML algorithms and software as a medical device. For over a decade, Bhavnani was the principal investigator of 90 clinical trials and patient care programs. These programs have enrolled over 30,000 patients in the US and in resource-limited areas. His team developed the SMART-FHIR integration interface for DHT data into EMRs for remote patient monitoring, remote therapeutic monitoring, and chronic care management, creating a real-world data platform to monitor the healthcare quality of DHT and ML devices in traditional and new consumer models of care delivery.
Catherine Liao – Chief Strategy Officer
Catherine Liao has served as our chief strategy officer since September 2022. Previously, Liao served as chief executive officer of Blumio, a pioneering medical device startup, from February 2016 to September 2022, where she led efforts in raising capital, formed a leadership and advisory board rich in knowledge spanning healthcare innovations, enterprise technology, and sensor technology. Her notable achievements include leading the commercialization of a groundbreaking medical radar sensor development platform, which garnered significant industry attention and was eventually acquired by Cardiex. Liao holds an MBA from Imperial College London and a Master of Science in Health Economics from the London School of Economics. These credentials underscore her deep and comprehensive insight into the intricacies of both the business world and the healthcare sector, demonstrating a balanced expertise critical for navigating and innovating within today’s complex healthcare economies.
Mark Gorelick, Ph.D. – Chief Product Officer
Dr. Mark Gorelick has served as Cardiex’s chief product officer since December 2020, bringing a wealth of experience from various leadership roles in the health and wellness technology sector. With an impressive tenure beginning in 2007, he has been at the helm as managing director of XPhys Technologies, a company at the forefront of developing innovative fitness, health, and wellness products. His strategic vision was further demonstrated through his role as vice-president of Digital Health, from 2018 to 2019, at Performance Lab Technologies, acclaimed for its software development prowess in the health sector. Further cementing his reputation in health technology, Gorelick served as the chief science officer at PAI Health (originally Mio Global) from 2015 to 2018, where he was instrumental in advancing health technology software solutions. Holding a BSc and MSc in kinesiology from Dalhousie University and a Ph.D. in biomedical science from University of Wollongong, Gorelick’s educational background underscores his deep-rooted understanding and innovative approach to biomedical science and kinesiology, reinforcing his invaluable contribution to our company and the broader health technology landscape.
This profile was written in collaboration with Couloir Capital.
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/bausch--lomb-announces-new-scientific-and-clinical-analyses-to-be-presented-on-bausch--lomb-infuse-silicone-hydrogel-daily-disposable-contact-lenses-during-the-american-academy-of-optometry-annual-meeting-301146326.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/bausch--lomb-announces-new-scientific-and-clinical-analyses-to-be-presented-on-bausch--lomb-infuse-silicone-hydrogel-daily-disposable-contact-lenses-during-the-american-academy-of-optometry-annual-meeting-301146326.html